Shear-wave elastography in breast ultrasonography: the state of the art
Article information
Abstract
Shear-wave elastography (SWE) is a recently developed ultrasound technique that can visualize and measure tissue elasticity. In breast ultrasonography, SWE has been shown to be useful for differentiating benign breast lesions from malignant breast lesions, and it has been suggested that SWE enhances the diagnostic performance of ultrasonography, potentially improving the specificity of conventional ultrasonography using the Breast Imaging Reporting and Data System criteria. More recently, not only has SWE been proven useful for the diagnosis of breast cancer, but has also been shown to provide valuable information that can be used as a preoperative predictor of the prognosis or response to chemotherapy.
Introduction
Elastography is an imaging modality based on tissue stiffness or hardness, which is analogous to clinical palpation with ultrasonography for a malignancy [1,2]. Unlike a physical examination, which allows only the subjective judgment of the stiffness of a lesion, elastography has the potential to quantify stiffness [2]. In breast ultrasonography, two elastographic techniques are popular and differ in the type of stress applied: strain and shear-wave elastography (SWE) [3]. Strain elastography produces an image based on the relative displacement of the tissue from an external (manual compression of the transducer) or patient source. It is difficult to measure the amount of the force or stress during compression, and the absolute elasticity cannot be calculated [2,3]. Meanwhile, SWE using the acoustic radiation force induced by the ultrasound push pulse generated by the transducer provides quantitative elasticity parameters, as well as displaying a visual color overlay of elastic information in real time [2,3]. In this article, the clinical applications and the current role of SWE in breast ultrasonography are reviewed.
Image and Data Acquisition
With shear waves that are induced by the acoustic radiation force and propagate transversely in the tissue, SWE can provide a semitransparent color-coded image displaying the shear wave velocity (m/sec) or elasticity (kPa) for each pixel in real time, because the speed of the shear waves can be measured and is linked to the Young modulus (kPa) [3-6]. To obtain SWE images of breast lesions, the rectangular field-of-view box of the system is set to include the lesion itself as well as the surrounding normal tissue; in this display, the tissue stiffness of each pixel in the image is shown as a semitransparent color map overlaid on the gray-scale image. The size of the rectangular region of interest (ROI) should be large enough to reveal any perilesional increased stiffness, because the maximum areas of stiffness in malignant lesions are almost always found in the area immediately adjacent to the lesions rather than in the lesion itself, and large enough to include normal fat tissue for the measurement of the lesion-to-fat ratio of elasticity (Fig. 1) [7,8]. In general, the range of the color scale within the ROI for breast lesions is from 0 (dark blue, indicating the lowest stiffness) to 180 kPa (red, indicating the highest stiffness), but the color scale can be adjusted to enhance the contrast of elasticity on the color map without any change in the absolute elasticity values [7].

A 38-year-old woman with a pathologically proven invasive ductal carcinoma.
A. Shear-wave elastography (right) and B-mode images (left) on split-screen mode show a 13-mm, irregular mass with red, heterogeneous elasticity. B. The mean, minimum, maximum, and standard deviation of elasticity values were measured in kPa by placing the region of interest (ROI) over the stiffest part of the lesion (circle). The elasticity ratio of the mass to the reference fat tissue was measured by placing a second ROI over the surrounding fat tissue (dotted circle). C. The ROI for measuring the elasticity value was placed to include the whole breast lesion and the stiffest part of the lesion.
To obtain high-quality SWE images, obtaining a good gray-scale image is essential before switching to the SWE mode because elastography images are often generated based on raw data from gray-scale images [9]. Since vibration energy is directly emitted from the probe, it is important to keep the angle of the probe perpendicular to the skin and the probe lightly touching the skin without consciously applying any vibration or compression [9,10]. If excessive compression or movement of the probe is applied, artifactual stiffness other than the target lesion is displayed as yellow to red, generally radiating from the skin surface or chest wall; this could be misinterpreted as high elasticity, even in a soft lesion. Using generous amounts of contact jelly and having the patient hold her breath may be effective in some cases to reduce artifacts [5,10]. Since it usually takes a few to several seconds for the color map of SWE to stabilize, depending on the case and the operator’s skill, the probe should be held still until the color display is completely stable before recording SWE images and measuring the elasticity of lesions in order to ensure that reliable results are obtained [9,10].
To measure the elasticity quantitatively in SWE for breast lesions, the most common practice is to place a 2- to 3-mm circular ROI over the stiffest part of the lesion, including the immediately adjacent stiff tissue or halo (Fig. 1). The elasticity parameters, including the mean (Emean), maximum (Emax), minimum (Emin), and standard deviation (ESD) of elasticity, are calculated automatically from the elasticity value of each pixel included in the ROI and displayed on the monitor of the ultrasound device in kPa or m/sec (Fig. 1). However, the size or shape of the ROI can be adjusted by the operator. In a previous study, the diagnostic performance of SWE, including its sensitivity, specificity, and accuracy, was influenced by the size of the ROI and varied across the elasticity parameters; therefore, the evaluation of all parameters with a 2-mm ROI was recommended [11]. In addition, a larger circular ROI encompassing the entire lesion as well as the stiffest part of the lesion can be used to quantify the elastic heterogeneity of a breast lesion by measuring ESD during SWE with good diagnostic performance (Fig. 1C) [12]. However, it is difficult to completely enclose irregular lesions with a circular ROI. To overcome this limitation, freehand ROI drawing techniques have been developed recently and are now commercially available (Fig. 2). The elasticity ratio (Eratio) of the breast lesion to the reference fat tissue can be measured by placing the first ROI over the stiffest part of the lesion, including the immediately adjacent stiff tissue or halo, and a second ROI in the fatty tissue of the breast (Fig. 1) [8]. Although the ROI for the reference fat can be set in various locations, since the diagnostic performance of Eratio was found not to be influenced by the measurement site of fat elasticity, images with a good quality should be obtained and the ROIs for the lesion and the surrounding fat should be set in areas without artifacts to avoid false-positive or -negative results [8]. Regarding the image acquisition planes, two orthogonal SWE images are recommended rather than a single SWE image, because breast tumors with intratumoral heterogeneity can appear to have different elasticity according to the selected imaging plane, just as they can show different morphology in gray-scale images [13]. It is recommended to select the higher elasticity score when two SWE images have a similar image quality but show a discordant finding, and to select the elasticity score of the SWE with the higher image quality when the image quality of the other SWE image is poor. To obtain much more elasticity information about a breast lesion in further planes, including the stiffest plane, 3-dimensional (3D) SWE can be used, which provides 3D volumetric color-coded elasticity maps of tissue stiffness in a single acquisition (Fig. 3) [14]. Unlike 2-dimensional SWE, in which the representative plane of the breast mass is chosen at the discretion of the operator and the stiffest portion of the tumor may be missed, 3D SWE can show the stiffest portion of the mass more easily and accurately [15].
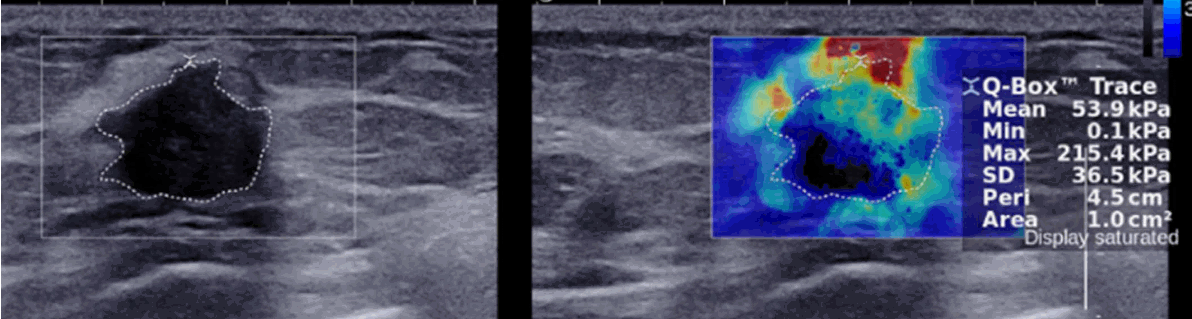
A 42-year-old woman with a pathologically proven invasive ductal carcinoma.
A freehand region of interest was drawn manually by tracing the border of the mass using shear-wave elastography to measure lesion elasticity.
Image and Data Interpretation
Quantitative Method
The breast tissue elasticity measured by SWE varies depending on the inherent tissue characteristics of the breast structures. For example, Emean measured in vivo by SWE ranges from 5 to 10 kPa in fatty tissue and from 30 to 50 kPa in breast parenchyma [16]. Pathologic conditions change breast tissue elasticity; in general, benign lesions tend to be harder than normal breast tissue but softer than malignant lesions [4]. In clinical practice, Emean, Emax, ESD, and Eratio are popular quantitative SWE parameters in the differential diagnosis of breast lesions visible on ultrasonography. However, no clear consensus exists regarding the best quantitative parameter or the most appropriate cutoff values. The ranges of cutoff values between benign and malignant breast lesions for each parameter with 2-mm ROIs have been reported to be as follows: 33.3-80 kPa (median, 59.35 kPa) for Emean; 46.7-93.8 kPa (median, 79.25 kPa) for Emax; 6.3-13.9 kPa (median, 9.8 kPa) for ESD; and 3.18-5.14 kPa (median, 3.56 kPa) for Eratio [3,7,8,12,14,15,17-27]. In the literature, Emean appears to be widely used in the diagnosis of breast lesions. However, ROI size should be considered when interpreting the results of Emean. Because Emean is the sum of all elasticity values of each pixel within the ROI divided by the number of pixels, its absolute value depends on the size of the ROI; that is, a higher Emean value for a smaller ROI is expected [28]. ESD, representing the average difference in elasticity value within the ROI to Emean, is also influenced by the ROI size [28]. As the size of the ROI changes, the sampling of the elasticity information becomes more variable, meaning that elastic heterogeneity will increase or decrease, particularly in a heterogeneous malignant lesion. For Emax, however, the stiffest part of the mass is always included within the ROI, regardless of the size of the ROI, and its absolute value is independent of the ROI size. For Eratio, the optimal measurement of reference fat elasticity is crucial, as fat elasticity is the denominator in calculating Eratio, and even a small difference in fat elasticity can cause a large difference in Eratio [8]. In addition, SWE anisotropy-the difference in lesion elasticity measured in two orthogonal planes could be used in the diagnosis of breast lesions [28]. Malignant lesions are more anisotropic than benign lesions.
Qualitative Method
From a color-coded elasticity map displayed in real time on SWE, the elasticity of breast lesions can be qualitatively evaluated for their diagnosis. In general, color map features can be visually assessed on the spot before measuring elasticity quantitatively, making the qualitative method more instantaneous. The color displayed for each pixel represents the elasticity information of the corresponding tissue, and once the color map features of the lesion are screened, an ROI can be placed over the stiffest part of the lesion to measure the elasticity quantitatively. For the qualitative assessment of breast lesions on SWE, a 4-color pattern classification based on color stiffness and heterogeneity was proposed by Tozaki and Fukuma [29]: in pattern 1, no difference from the color around the lesion is observed at the margin of the lesion or in its interior (coded blue homogeneously); in pattern 2, a color that differs from the color around the lesion is observed at the margin or in the interior of the lesion, but it extends beyond the lesion and continues vertically in cords on the cutaneous side or the thoracic wall side (vertical stripe pattern artifacts); in pattern 3, a localized colored area is present at the margin of the lesion; and in pattern 4, colored areas are present in the interior of the lesion heterogeneously (Fig. 4) [29,30]. Pattern 2 is a unique artifact frequently observed during SWE examinations, with reported ranges from 7% to 24% [24,29-31]. Patterns 3 and 4 are characterized by peripheral increased stiffness (the “stiff rim sign”) and heterogeneous color map features, suggestive of malignancy [7]. The stiff rim sign may be caused by (1) a desmoplastic reaction or the infiltration of cancer cells into the interstitial tissues or the intraductal component; or (2) internal low shear wave amplitude and/or noise as well as peripheral high-speed shear wave in the lesion caused by attenuation of the energy of the shear wave in the periphery of the lesion [7,32]. Elastic heterogeneity is regarded to represent histologic heterogeneity of malignant lesions that are in part more cellular due to lymphocytic infiltration and/or in part more necrotic [12]. Berg et al. [19] proposed qualitative E values, Ecol (a 6-point color score of maximum elasticity: red, orange, green, light blue, dark blue, or black), Ehomo (homogeneity of elasticity: very homogeneous, reasonably homogeneous, or heterogeneous), and Esha (lesion shape: oval, round, or irregular).

Four color patterns on shear-wave elastography.
A. Pattern 1: no difference from the color around the lesion is observed at the margin of the lesion or in its interior. B. Pattern 2: a color that differs from the color around the lesion is observed at the margin or in the interior of the lesion, but it extends beyond the lesion and continues vertically in cords on the cutaneous side or the thoracic wall side. C. Pattern 3: a localized colored area is present at the margin of the lesion. D. Pattern 4: colored areas are present in the interior of the lesion heterogeneously.
In the fifth edition of the Breast Imaging Reporting and Data System (BI-RADS), descriptors for qualitative elasticity assessment were added: soft, intermediate, and hard. It is emphasized that a soft elastogram must not supersede morphologic analysis. Stiffness as a feature of malignant masses may be considered along with their much more important morphologic characteristics [33].
Clinical Applications of SWE
Differentiation of Benign and Malignant Breast Lesions
The diagnostic performance of SWE is good for differentiating between benign and malignant breast lesions. In literature, the median sensitivity, specificity, and area under receiver operating curve of each quantitative parameter have been reported as follows: 88.6% (range, 81.0% to 95.8%), 89.9% (range, 68.2% to 93.8%), and 0.932 (range, 0.788 to 0.974) for Emean; 90.3% (range, 60.9% to 97.0%), 81.8% (range, 77% to 100%), and 0.931 (range, 0.741 to 0.961) for Emax; 89.2% (range, 83.1% to 91.1%), 91.1% (range, 67.9% to 93.0%), and 0.899 (range, 0.761 to 0.964) for ESD; and 88.0% (range, 71.4% to 96.7%), 93.7% (range, 90.6% to 100%), and 0.952 (range, 0.917 to 0.987) for Eratio, respectively [3,6,7,12-15,17,21-26,32,34-39]. The best-performing SWE parameter in diagnosing breast lesions has been reported to be Emax or Eratio [15,19,25,26,38,40].
The combination of SWE with conventional B-mode ultrasound increases the diagnostic performance for breast lesions, compared with conventional B-mode ultrasound alone [3,4,7,18,19,25,26,34]. In a meta-analysis, the sensitivity, specificity, and the area under receiver operating curve were reported as follows: 97.1% (95% confidence interval [CI], 94.1% to 98.6%), 80.1% (95% CI, 73.3% to 85.6%), and 0.96 (95% CI, 0.94 to 0.97) for both techniques in combination; and 94.9% (95% CI, 88.1% to 97.9%) and 55.2% (95% CI, 26.4% to 80.9%), and 0.93 (95% CI, 0.90 to 0.95) for conventional B-mode ultrasound alone, respectively [41]. The use of SWE as an adjunct to conventional B-mode ultrasound can increase diagnostic confidence and improve patient management. More specifically, SWE features can help reclassify BI-RADS category 3 or 4a lesions by morphologic criteria on conventional B-mode ultrasound. In the BE1 multinational prospective study, the use of SWE features to downgrade BI-RADS category 4a lesions to follow-up or to upgrade BI-RADS category 3 lesions to biopsy improved specificity from 61.1% for B-mode ultrasound alone to 78.5% by applying visual color stiffness or 77.4% by using Emax, without changing the sensitivity [19]. This result is underscored by other studies suggesting that the addition of SWE reduced the number of unnecessary biopsies by enabling a switch to follow-up in benign BIRADS category 4a lesions [3,13,25,30]. In addition, using the same criteria of the likelihood of malignancy as the BE1 study group, SWE helped downgrade benign BI-RADS category 3 lesions to category 2 and reduce the number of unnecessary initial short-term follow-up visits, especially when Emax was ≤20 kPa or the visual color stiffness was black to dark blue [42]. In clinical practice, SWE frequently helps radiologists have greater confidence when its findings concur with conventional ultrasound findings [43].
Prediction of Breast Cancer Prognosis
Breast cancer is considered to be a group of heterogeneous diseases, in terms of morphology, clinical course, and response to treatment [44]. To characterize breast cancer and predict its prognosis accurately is the mainstay of successful treatment. Clinicopathological features such as histological type, tumor size, histological grade, the presence of lymph node metastasis, and lymphovascular invasion have been well established as prognostic factors of breast cancer. Through gene expression profiling, breast cancer has been divided into four subtypes: luminal A, luminal B, human epidermal growth factor receptor 2 (HER2)-enriched, and basal cell-like subtypes, showing different clinical outcomes [44]. As a surrogate for molecular classification, immunohistochemical profiling for the expression of the hormonal receptors HER2 and Ki-67 has been suggested, and has been shown to be associated with different clinicopathological features, prognosis, and treatment responses [44]. Regarding SWE, a large invasive size, high nuclear grade, high histologic grade, and lymphovascular invasion were reported to be associated with increased stiffness of invasive breast cancer [44-47]. Estrogen receptor negativity, progesterone receptor negativity, p53 positivity, and Ki-67 positivity were significantly associated with a higher Eratio, and triple-negative and HER2-positive tumors showed greater stiffness than estrogen receptor-positive tumors [46,47]. Interestingly, some aggressive tumors, such as high-grade cancers and triple-negative tumors, are likely to be assessed as BI-RADS category 3 in B-mode ultrasound, but SWE may provide additional information for diagnosing those benign-looking malignancies [47].
Predicting the axillary lymph node status in patients with newly diagnosed breast cancer is an integral component of breast cancer management, including the staging, treatment plan, and prognosis [48]. Ultrasonography has been performed for the noninvasive preoperative evaluation of the axillary nodal basin because it is widely available and easily incorporated into the standard workup for breast cancer patients [49]. Ultrasonographic criteria based on size or morphologic characteristics have shown variable diagnostic performance for metastatic lymph nodes [50]. In two previous studies-one in vivo and the other ex vivo-of SWE for sentinel axillary lymph nodes in patients with breast cancer [51,52], greater axillary lymph node stiffness was correlated with the risk of metastasis, and the high specificity of lymph node cortical stiffness can be complementary to B-mode ultrasound for decision-making regarding fine-needle aspiration biopsy. In future, further investigations of larger populations are needed to validate these results and apply SWE in clinical practice.
Prediction of the Response of Breast Cancers to Neoadjuvant Chemotherapy
Neoadjuvant chemotherapy (NAC) has been applied as an established treatment strategy for tumor down-staging in patients with breast cancer who would not be optimally treated by immediate surgery [53]. A complete pathologic response after NAC may be a predictor of a low risk of subsequent recurrence and longer disease-free survival [54,55]. However, the response to NAC can be quite variable. The early and accurate prediction of responsive and resistant tumors to NAC is crucial to avoid futile chemotherapy and to guide more effective treatment strategies, such as modifying the chemotherapy regimen or optimizing the timing of surgery in nonresponsive patients [53,55]. A clinical examination combined with conventional imaging modalities has not yet become sensitive or specific enough to predict pathologic responses to NAC. Interestingly, recent studies have reported that increased stromal gene expression may be a predictor of response to NAC and that tumors with disorganized stroma had a reduced pathologic response to NAC, which means that stromal factors as well as tumor factors are important in predicting the response to NAC [53,56,57]. Considering that tumor stiffness is related to the collagen content in the stroma, stromal stiffness measured by SWE may be useful as an imaging biomarker for stromal structural abnormalities and the response to NAC [20,53]. Tumor elasticity measured by SWE before NAC had a significant relationship with a subsequent reduction in the cellularity of the primary tumor in response to NAC [20]. During NAC, the relative changes in tumor elasticity showed a significant correlation with the response to NAC, and the second NAC cycle was recommended as the optimal time point for performing SWE evaluations to reduce the chance of unnecessary cytotoxic exposure or to perform surgery in patients with NAC resistance [53-55,58]. A previous study suggested the optimal relative change in the stiffness threshold to be -36.1%, with a sensitivity of 73% and a specificity of 86%, to distinguish between responders and nonresponders after two cycles of NAC [55].
Pitfalls
Although quantitative elasticity information obtained by SWE in addition to B-mode ultrasound has improved diagnostic performance, false results have been reported in 6.4%-36.6% of cases, in which the imaging results did not correspond to the pathologic results [59]. Specifically, the false-positive rates of benign masses (53% using qualitative analysis and 22%-37% using quantitative analysis) were reported to be higher than the false-negative rates of malignant masses (8% using qualitative analysis and 6%-10% using quantitative analysis) [23,27]. Considering that benign breast lesions showing false-positive SWE findings were significantly larger, the false-positive results can be explained by the size of the breast mass, as larger masses are likely to cause the probe to be unevenly applied to the skin above the masses, which could hinder adequate image acquisition [23]. Other lesion-related factors contributing to false diagnoses in SWE may include the presence of pure in situ disease, smaller malignant masses, malignant masses with a circumscribed margin and an abrupt lesion boundary, and the grade of invasive disease [23,27,60]. Aside from the intrinsic tumor characteristics, patient-related or clinical factors associated with false elastography features include the mode of detection (symptomatic vs. mammography screening), age at diagnosis, breast thickness, lesion depth, distance from the nipple to the lesion, and the quality of the image [23,27,59,60]. When SWE examinations are performed and interpreted for breast masses, investigators should take into consideration the clinical and lesion-related factors that are associated with inaccurate elastography findings.
Summary
Breast elastography is now an adjunct tool in breast ultrasonography. It is easily performed in clinical practice, adding only a short amount of time to breast ultrasonography. To ensure the best possible performance of SWE in the diagnosis of breast cancer, the technique should be optimized to acquire high-quality images, and practitioners should properly interpret the acquired images and data. One of the best applications of SWE is the characterization of breast masses categorized as BI-RADS category 3 and 4a, in order to attempt to reduce unnecessary breast biopsies. In addition, SWE can provide additional information on predicting breast cancer prognosis and response to NAC. However, the possibility of false-positive and false-negative results should be considered during interpretation. An adequate understanding of the features of each elastography method allows proper imaging and diagnosis to be carried out, confirming that elastography is indeed a clinically useful tool.
Notes
No potential conflict of interest relevant to this article was reported.

