Morphological alterations of the tendon and pulley on ultrasound after intrasynovial injection of betamethasone for trigger digit
Article information
Abstract
Purpose
The aim of this study was to elucidate whether intrasynovial corticosteroid injections for trigger digit reduced the volume of the tendon and pulley on high-resolution ultrasonography.
Methods
Twenty-three digits of 20 patients with trigger digit were included. Each affected finger was graded clinically according to the following classification: grade I for pre-triggering, grade II for active triggering, grade III for passive triggering, and grade IV for presence of contracture. Axial ultrasound examinations were performed before an intrasynovial corticosteroid injection and at an average of 31 days after the injection. The transverse diameter, thickness, and cross-sectional area of the tendon and the thickness of the pulley were measured by two independent, blinded researchers.
Results
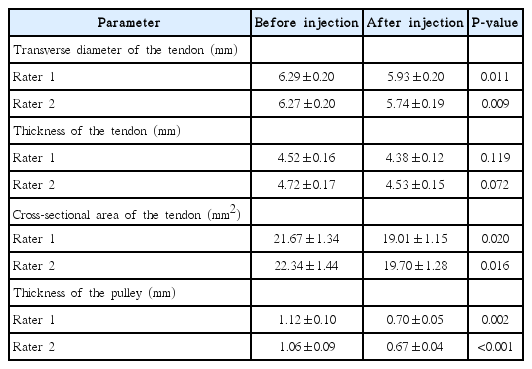
At least 1 grade of improvement was achieved in this study group by the time of the second examination. The transverse diameter and cross-sectional area of the tendon and the thickness of the pulley significantly decreased (P<0.05).
Conclusion
The injection of a single dose of betamethasone improved clinical symptoms by reducing the volume of both the tendon and pulley, which may be related to the fact that tendon and pulley ruptures are delayed by corticosteroid injections.
Introduction
Although trigger digit is a relatively common complaint in orthopaedic clinics, its cause is not fully understood. The main symptoms are snapping of the digit and tenderness over the first annular (A1) pulley. It is thought to be caused by stenosing tenosynovitis or tendon entrapment at the A1 pulley induced by a mismatch between the size of the pulley and the volume of the flexor tendons [1].
Many cases of symptomatic trigger digit can now be managed non-surgically with corticosteroid injections, which have been found to be effective in 64%-97% of cases [2-7]. However, postinjection changes in the pulley and tendon have not been fully investigated. Moreover, concerns have been raised about the deteriorative effect of corticosteroids on connective tissue.
Recently, high-resolution ultrasonography with a high-frequency transducer has enabled morphological changes in small structures in the finger to be observed [8]. Serafini et al. [9] reported that flexor tendon thickening was the main cause of trigger digit and suggested that a reasonable cut-off for pathological findings was a 20% increase in tendon thickness compared with the contralateral tendon. Guerini et al. [10] reported thickening and hypoechogenicity of the A1 pulley in all patients with trigger digit, and hypervascularity of the A1 pulley in 91% of patients. In addition, Sato et al. [11] reported that the tendon tended to be thicker under the A1 pulley in accordance with the clinical grade of trigger digit, and that thickening of the volar plate affected continuous triggering. Although several pathophysiological mechanisms have been suggested, trigger digit can be reliably assessed by ultrasonographic measurements of the pulley and tendon.
Hence, this study employed high-resolution ultrasonography to investigate morphological changes in the tendon and the pulley after a single corticosteroid (betamethasone suspension) injection in patients with trigger digit.
Materials and Methods
All study procedures were conducted in accordance with the 1975 Declaration of Helsinki, as revised in 2008 [12], and this study received approval from the Institutional Review Board. Informed consent to be included in this study was obtained from all patients. Consecutive patients clinically diagnosed with trigger digit at our institution between April 2011 and March 2015 were recruited, while patients with rheumatoid arthritis, a prior local corticosteroid injection, severe diabetes mellitus meaning that a local corticosteroid injection might be harmful, a history of infectious disease or major trauma in the same digit, or who were unwilling to participate in this study were excluded from this study. We used radiographs to confirm that none of the patients had a bony deformity, calcium deposition, or osteoarthritis in the digit. Twenty-three digits (five thumbs, one index, nine middle, and eight ring fingers) of 20 patients (seven males and 13 females; mean age, 64.0±9.7 years; range, 44 to 86 years) with trigger digit were ultimately included in this study after the exclusion of a patient who did not show clinical improvement after the treatment. Each affected finger was graded clinically according to signs of triggering: grade I (pretriggering) was defined by pain and a history of catching, but without demonstrable catching on the physical examination; grade II (active triggering) involved demonstrable catching, but the ability to actively extend the digit; grade III (passive triggering) was assigned to patients requiring passive extension or presenting an inability to engage in active flexion; and grade IV (contracture) was assigned to patients who showed fixed flexion contracture of the proximal interphalangeal joint [13].
All ultrasound examinations and patient management were performed by one author (M.T., 11 years of experience of hand surgery and >20 years of orthopaedic surgery). An axial view using high-resolution ultrasonography (EUB-7500 with a linear-array high-frequency transducer, Hitachi Medical Corp., Tokyo, Japan) was obtained from the volar aspect at the metacarpophalangeal (MP) joint with minimum pressure. The patient was relaxed in the supine position with the affected forearm positioned in supination, the wrist in neutral, and the MP joint in extension. The transducer was placed perpendicular to the tendon, such that the tendon was characterised by its hyperechoic fibrillar pattern and the surrounding fluid distension was maximal. After confirming thickening of the A1 pulley in comparison with that of the adjacent non-affected digit or that of the contralateral digit, 2.5 mg (0.5 mL) of an injectable suspension of betamethasone (Rinderon, Shionogi Co. Ltd., Osaka, Japan) with 10 mg (0.5 mL) of procaine was administered into the intrasynovial space beneath the affected pulley. The injection was made with a 27-gauge needle in a relief-of-resistance manner and with palpation of the distal tendon sheath to monitor ballooning during the injection. Patients were advised to exercise the affected digit after the injection. An ultrasound examination was repeated in the same manner as the initial examination at an average of 31 days (range, 26 to 39 days) after the injection. The axial images presenting maximal surrounding fluid distension both before and after the injection were chosen by the examiner (M.T.) for later measurements (Fig. 1). Two independent raters, both with >8 years of experience of orthopaedic surgery, who were blinded to all medical information about the patients, measured the selected parameters on the chosen image. The transverse diameter, thickness, and cross-sectional area of the tendon and the thickness of the pulley were measured (Fig. 2). The two raters measured these parameters on an independent computer with ImageJ software (ver. 1.46r, NIH, Bethesda, MD, USA) at almost the same time. Statistical analyses were performed using SPSS Statistics for Windows, ver. 22.0 (IBM Corp., Armonk, NY, USA). Linear regression was performed and intraclass correlation coefficients (ICCs) were calculated to quantify the level of agreement between the two raters. All data are averages from the two raters. Data are expressed as mean and standard error of the mean, unless otherwise mentioned. The paired t test was used to compare findings from the two ultrasound examinations. P-values <0.05 were considered to indicate statistical significance.

A 70-year-old man with grade III trigger finger at the left 4th finger.
A. Longitudinal view on ultrasonography over the palmar aspect of the affected metacarpophalangeal joint refers the positional relation of the axial images. Dotted lines indicate the corresponding axial planes shown in C and D. Scale bar=10 mm. B. The same view with A shows the A1 and A2 pulleys (shaded area) over the tendon (T), volar plate (V), metacarpal head (M) and proximal phalanx (P). C, D. Consecutive axial images show the distal, less thick pulley (C) and the thickest pulley (D). Hypoechoic fluid distension was maximal at the level of D, E. Ultrasonography at the same level as D conducted 26 days after the injection shows decreased thickness of the tendon (from 3.78 to 3.41 mm), cross-sectional area of the tendon (from 18.52 to 16.15 mm2), and thickness of the pulley (from 0.922 to 0.533 mm), but no decrease in the transverse diameter of the tendon (from 5.59 to 5.81 mm) in this case.

Axial view for the measurements.
A-D. An axial view on ultrasonography is used in this study to measure four parameters: the transverse diameter of the tendon (A, dotted line), the thickness of the tendon (B, dotted line), the cross-sectional area of the tendon (C, dotted line), and the thickness of the pulley (D, arrows). The tendon shows a hyperechoic fibrillar pattern, while hypoechoic fluid distension is maximal.
Results
In this study population, two digits were grade II, 19 were grade III, and two were grade IV prior to the injection. At the second ultrasound examination, at least one grade of improvement was achieved in this study group: 14 digits were grade I and nine were grade II. Thus, overall improvement to the point that triggering was absent occurred in 61% of the population (14 of 23 digits). No patients complained of any side effects after the injection. All measurement values obtained from the two raters agreed well. This was reflected in the results of regression analyses showing good inter-rater agreement (n=23; P<0.001 for all parameters; r2 =0.853-0.965). Absolute numeric agreement was excellent (ICC, 2, 1; range, 0.899 to 0.980), confirming the validity of the measurements made using ImageJ.
After the injection, the transverse diameter and cross-sectional area of the tendon and the thickness of the pulley significantly decreased, as assessed by both raters (P<0.05 for these three parameters) (Table 1). The thickness of the tendon also decreased after the injection, although it was not significant, according to both raters (P>0.05).
Discussion
This study demonstrated that measured values of tendon volume and pulley thickness decreased approximately 1 month after a single betamethasone injection into the intrasynovial space of symptomatic tendons and pulleys. The thickness of the pulley is usually quite small (<1 mm for a normal pulley). The recent development of high-resolution ultrasonography with a high-frequency transducer has enabled the detection of subtle changes in the thickness of the pulley.
Our results regarding changes in thickness of the pulley after betamethasone injection largely concur with those reported by Miyamoto et al. [14], who reported that increased thickness and stiffness of the A1 pulley were causes of snapping in the trigger digit, and that the thickness and stiffness of the A1 pulley decreased 3 weeks after triamcinolone acetonide injection. Their report, however, reported no reduction of the cross-sectional area of the tendon after the injection. This divergence in the results regarding the cross-sectional area of the tendon may be due to differences between the studies, including the corticosteroid that was applied and the timing of the ultrasound examination. In the previous study, the researchers applied 10 mg of triamcinolone acetonide to the tendon sheath, whereas we used 2.5 mg of betamethasone, which is 5- to 6-fold more potent per milligram in terms of its antiinflammatory effects than triamcinolone. The second ultrasound examination was performed 3 weeks after the injection in the previous study, whereas we performed the second examination at an average of 1 month after the injection.
The therapeutic effects of a corticosteroid injection into the trigger digit may vary. Reduction of the volume might be achieved through a decrease in tissue oedema, which is a pharmacological effect of corticosteroids [15,16]. Several experimental studies have shown that corticosteroid-induced tendinopathies occurred through reduced synthesis of collagen type I and proteoglycans, as well as decreased tenocyte proliferation, differentiation, viability, and metabolism [17-22]. It has also been shown that under specific conditions, corticosteroids increase the synthesis of matrix metalloproteinase (MMP)-1 and MMP-13, both of which can cleave collagen type I [23,24]. Considering these findings, it appears that corticosteroids may negatively affect the tendon and the pulley, although the corticosteroid dosage and the timing of the examination should be considered.
Despite the controversy over the effects of corticosteroids on the tendon, it is certain that reduction of the pulley thickness is a major mechanism through which corticosteroid injections affect trigger digit. In contrast to the tendon, which consists of a dense collagen matrix, the pulley has been shown to have a loose connective layer in addition to a dense normal connective layer [25]. In addition, an innermost layer composed of irregular connective tissue has been reported in pathological conditions such as trigger digit. These less dense elements are thought to be more susceptible to corticosteroids than dense tissues, such as tendons. We speculate that the effects of corticosteroids on the tendons emerge later than the effects on the pulleys due to the difference in the density of the connective tissue.
Delayed ruptures of the flexor tendon or pulley after a corticosteroid injection have been reported in the literature [26-29]. In these reports, multiple corticosteroid injections were administered, and the rupture occurred many months after the first injection. Even though a causative link between corticosteroid injection and tendon or pulley rupture cannot be made, all authors have recommended that repetitive intratendinous or intrasynovial injections should be avoided due to the known attritional effects of corticosteroids on collagen. These reports indicate that the deteriorative effects of corticosteroid on the tendon last for a long period, and that careful observation is required for many months after corticosteroid injection.
This study has several limitations. First, the sample size was relatively small, although we addressed a commonly occurring pathological condition. However, the design of the study, in which two independent, blinded raters measured the ultrasound parameters, may compensate for the small sample size. Second, this study did not have a control group. Since corticosteroid injection for trigger digit is a widely accepted treatment and the patients came to our institution seeking treatment with a definitive effect, we did not design a placebo control group. Finally, we did not examine the parameters on ultrasound at a later time point, which would be another issue of interest. A goal of our future research is to address the long-term effects of betamethasone injections.
In summary, we have shown, using axial high-resolution ultrasonography, that a single dose of intrasynovial betamethasone injection for trigger digit improved clinical symptoms by reducing not only the thickness of the pulley, but also the volume of the tendon. Further studies are needed to understand the deteriorative effects of corticosteroids on tendinous tissue and to identify safe doses for corticosteroid injections.
Notes
No potential conflict of interest relevant to this article was reported.