Primary squamous cell carcinoma of thyroid gland with local recurrence: ultrasonographic and computed tomographic findings
Article information
Abstract
Primary squamous cell carcinoma of the thyroid gland (PSCCT) is a rare malignancy that presents with advanced disease and poor prognosis. It is difficult to diagnose PSCCT in its early stage because of its rarity and lack of typical imaging findings. We experienced an elderly woman with PSCCT confirmed by surgery. Although preoperative fine-needle aspiration revealed no malignancy, surgical resection was performed because the ultrasonogram showed diffuse microcalcifications, which suggested malignancy, and clinically, the mass grew rapidly to compress the trachea. Local tumor recurrence was noted at 3 months after surgery. Surgical resection or repeat biopsy should be considered if a cytologically benign thyroid mass shows imaging or clinical features of malignancy.
Introduction
Primary squamous cell carcinoma of the thyroid gland (PSCCT) is extremely rare and constitutes less than 1% of thyroid malignancies. PSCCT is characterized by a very aggressive course with a poor prognosis, and local tumor recurrence after surgery is common. There have been numerous reports in the English literature on imaging features of malignant thyroid nodules, but most of these have addressed papillary carcinomas. Herein, we describe the ultrasonography (US) and computed tomographic (CT) findings of PSCCT in the case of 70-year-old woman who presented with a palpable neck mass.
Case Report
A 70-year-old woman presented to our hospital with a large neck mass that had been observed a few months previously. Physical examination revealed a 5 cm×4 cm hard nodule in the left side of the neck without tenderness. The patient had undergone fine needle aspiration (FNA) of the neck mass at an outside hospital six days earlier and the cytologic report indicated benign follicular cells and colloid, consistent with a benign follicular nodule (Bethesda category II). Initial laboratory data revealed hypothyroidism, with a free thyroxine (fT4) level of 0.815 ng/dL (normal range, 0.93 to 1.7 ng/dL) and thyroid stimulating hormone within the normal range. Her chest radiography showed upper tracheal deviation to the right side due to the extrinsic mass effect. She did not complain of any respiratory distress or dysphagia and her pulmonary function test was within normal range. She had no history of smoking or radiation exposure to the neck. US findings showed a 5.0 cm×4.2 cm×6.1 cm sized, well-defined, lobulating, heterogeneously hypoechoic solid mass with suspicious microcalcifications in the left thyroid gland, and the thyroid capsule seemed to be intact (Fig. 1A, B). The background echotexture of the thyroid gland was heterogeneous, and these findings suggested diffuse thyroid disease such as Hashimoto thyroiditis. Several small lymph nodes (LNs) were noted without typical suspicious features, but two LNs were slightly enlarged up to 6 mm in short diameter in the right neck at level IV and in the left supraclavicular area. Using a freehand technique, repeated US-guided FNA was performed for the left thyroid mass immediately after neck US. Cytologic examination by FNA revealed sheets of follicular cells and a few macrophages, favoring nodular hyperplasia (Bethesda category II) (Fig. 1C). Some lymphoid cells were also found in the background of the FNA, and chronic lymphocytic thyroiditis was suggested. We performed additional FNA for left supraclavicular LN to rule out metastasis, the cytologic result revealed reactive nodal hyperplasia. Despite benign results from performing FNA twice, a left hemithyroidectomy was planned to confirm diagnosis because the mass showed relatively rapid growth, suspicious US findings, and airway compression. Ten days after neck US, preoperative contrast-enhanced CT was performed, and there was a well-defined, heterogeneously enhancing solid mass with a large central nonenhancing portion in the left thyroid gland (Fig. 1D, E). Despite the mild bulging contour of the mass, the capsule seemed to be intact, and there was no evidence of LN metastasis.
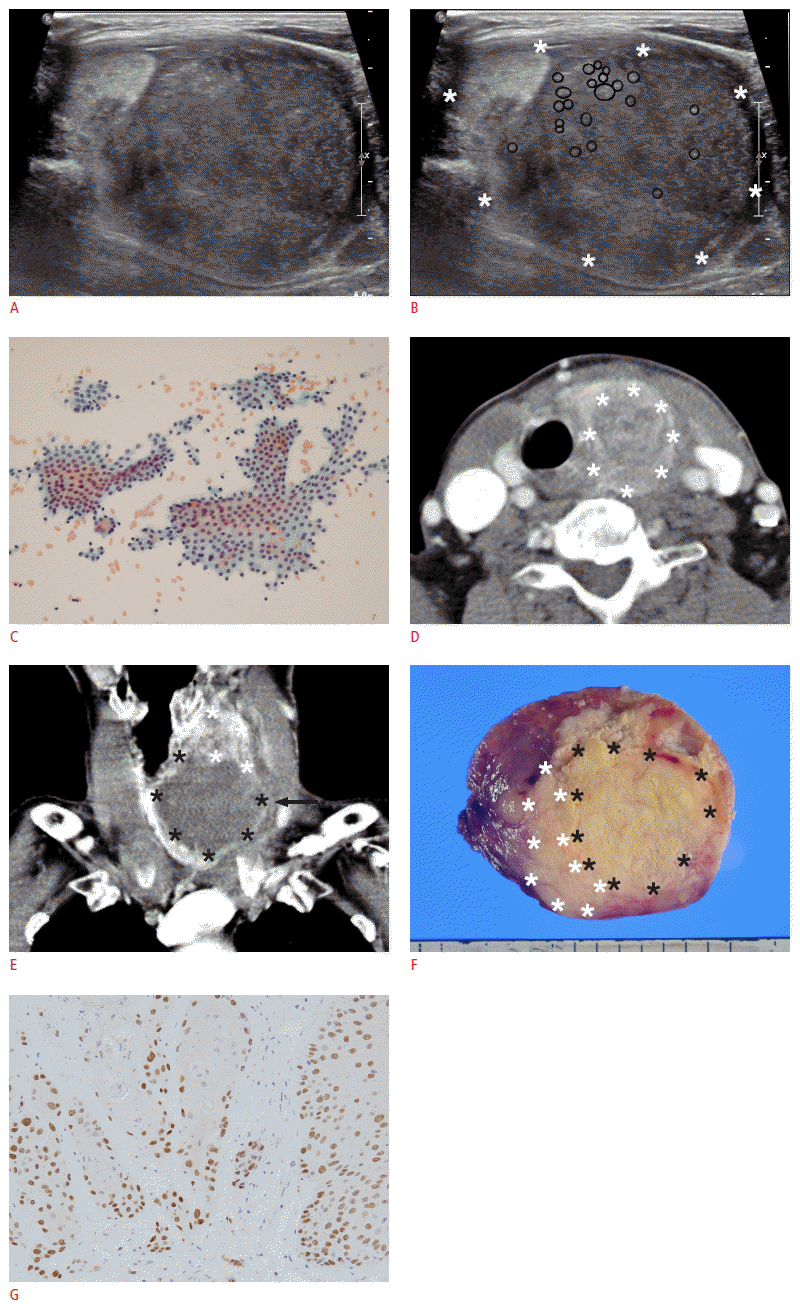
A 70-year-old woman with primary squamous cell carcinoma (SCC) of the thyroid gland in the left neck.
A, B. Longitudinal gray scale ultrasonography of the left thyroid gland shows a 5.0 cm×4.2 cm×6.1 cm, oval shaped, well-defined lobulated heterogeneously hypoechoic solid mass (asterisks) with suspicious microcalcifications (circles) mostly located in the central portion. C. Preoperative fine needle aspiration cytology of the mass demonstrates a few sheets of benign-looking follicular cells and macrophages, favoring nodular hyperplasia (H&E, ×200). D, E. A contrast-enhanced computed tomography scan shows the neck mass with a peripheral heterogeneously enhancing portion (white asterisks) and central nonenhancing necrotic portion (black asterisks). Although the left thyroid gland shows a mild focal bulging contour (arrow) due to the mass, the thyroid capsule seems to be intact. F. The gross specimen of the left thyroid mass shows a well-defined, round mass consisting of a gray-white keratinous material in the upper peripheral portion (white asterisks) and a large yellowish necrotic portion (black asterisks) in the central portion. G. Immunochemistry shows SCC cells positive for p63 (p63 immunostaining, ×200).
The operation was done, and total thyroidectomy with radical neck dissection was additionally performed because the intraoperative frozen biopsy suggested malignancy. The gross specimen showed a well-defined, ovoid mass in the left thyroid gland, which consisted of large central necrotic portions and peripheral graywhite, keratinous materials, measuring 6 cm×6 cm (Fig. 1F). Microscopic examination revealed nests of tumor cells infiltrating a desmoplastic background besides underlying lymphocytic thyroiditis. There was no extrathyroidal extension and such cells showed positive immunoreactivity for p63, p53, and Ki-67 (Fig. 1G). The histopathologic diagnosis was squamous cell carcinoma (SCC) without regional LN metastasis (pT3N0). Additional examinations including chest CT, a bone scan, positron emission tomographycomputed tomography (PET-CT), and endoscopy were performed to rule out metastatic SCC of the thyroid gland from another primary origin, but there were no discernible abnormalities, and the final diagnosis was PSCCT. Adjuvant radiotherapy was planned, but the patient refused treatment and was discharged 6 days after surgery.
After 3 months, she revisited our hospital complaining of a newly growing mass in her neck that had been present for a few weeks without any associated respiratory symptoms. On US, an irregularly shaped, hypoechoic mass 3.5 cm×5.5 cm in size in the surgical site of the left neck was found (Fig. 2A). Several ovoid, enlarged LNs with fatty hilum were found in the left lateral neck, from level II to the supraclavicular fossa. A chest CT scan showed a large, wellmarginated, nonenhancing solid mass in the left thyroid fossa, and PET-CT showed increased 18F-fluorodeoxyglucose (FDG) metabolism of the mass (Fig. 2B, C). The left-sided neck LNs showed no FDG metabolism, and there was no evidence of distant metastases on the PET-CT or bone scan. She refused US-guided FNA of the neck mass and was referred to another hospital, as requested.
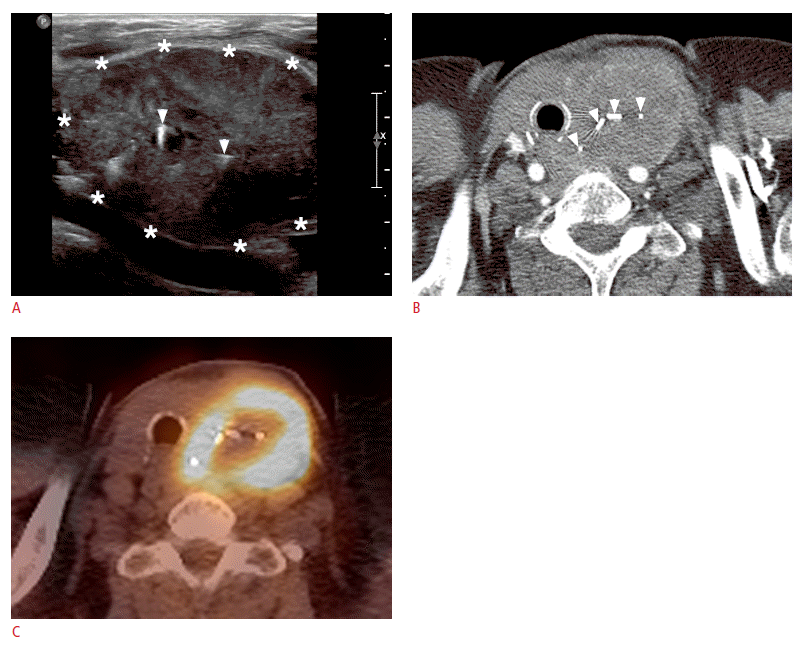
A mass suggestive of recurred primary squamous cell carcinoma of the thyroid gland (PSCCT) in left neck 3 months after surgery.
A. Longitudinal grayscale ultrasonography of the neck shows a huge, well-defined, heterogeneously hypoechoic mass (asterisks) with diffuse microcalcifications in the left neck postoperative site. Two bright linear hyperechogenicities in the mass (arrowheads) represent surgical clips. B. A contrast-enhanced computed tomography scan shows a large, well-defined, nonenhancing isodense mass containing several surgical clips (arrowheads) in the left postoperative site. C. A positron emission tomography-computed tomography scan shows increased 18F-fluorodeoxyglucose metabolism in the left neck mass, suggestive of recurred PSCCT.
Discussion
PSCCT is a rare neoplasm that is characterized by a very aggressive clinical course and poor prognosis. In general, there is no squamous epithelium in the normal thyroid gland. Thus, the origin of primary SCC within the thyroid gland remains controversial and several hypotheses include (1) squamous metaplasia superimposing on an underlying pathology such as Hashimoto thyroiditis, (2) squamous differentiation in papillary carcinoma or anaplastic carcinoma, (3) an embryonic rest, in which squamous cell cancer develops in remnants of the ultimobranchial body or thyroglossal duct [1-3]. Our case well supports the cell metaplasia theory, as this PSCCT arose in a background of lymphocytic thyroiditis.
The classic triad of features of PSCCT have been described in the literature [1,4] as follows: (1) a rapidly enlarging mass observed in older patients that behaves like anaplastic carcinoma, (2) the mass may be associated with other thyroid malignancies, and (3) histological features of intercellular bridges and keratin. Although several reported cases of PSCCT have been diagnosed by FNA [5,6], the cytological findings are typically nonspecific, and clinical correlation is needed for correct diagnosis [4,7]. In our case, the repetitive cytological results suggested a benign pathology, but postoperative histopathologic examination revealed malignancy. In general, the best known cause of FNA misdiagnosis is a sampling error owing to the inhomogeneity of the primary tumor. We presume the discordant results of our case could also be due to a sampling error because the histology of the resected thyroid gland showed variegated cellular components including fibrous tissue elements, as follows: (1) an area of normal follicles with colloid, (2) other portions showing lymphocytic infiltration with a germinal center, (3) another part of the nodule with SCC, and (4) a large necrotic portion of keratin material. Nevertheless, it is interesting that two separate FNA applications showed same the benign result by chance. According to Sahoo et al. [1], another possible cause of FNA misdiagnosis of SCCT is that carcinoma cells were firmly held by a desmoplastic and fibrotic reaction within the tumor and were consequently not picked up by FNA, but the prevalence of this phenomenon has not been determined.
The imaging findings of PSCCT have seldom been published. Regarding US findings, PSCCT has been reported to be a nodule with eggshell calcification and peripheral soft tissue [8], or as a slowly growing, irregularly marginated hypoechoic solid nodule [9].
In our case, PSCCT presented as a large, well-defined, lobulated, heterogeneously hypoechoic mass with diffuse microcalcifications on US. These microcalcifications are a well-known US feature of malignant thyroid nodules, but in our case, there were no observable microcalcifications in the pathologic findings. We reviewed the pathologic slide again to search for microcalcifications, but there was no calcification at all. We presumed this discordancy was probably due to the location of microcalcifications, the central necrotic portion, which could have been washed out during the preparation process of the pathologic specimen. We tried to perform specimen radiography to confirm the presence of microcalcifications beyond the necrotic portion, but all of the viable tumor portion had already been made into a slide and there was no remnant specimen block.
Regarding CT findings, there have been only a few case reports, and most of them have shown a huge mass containing focal cystic changes or coarse and curvilinear calcification, resulting in airway compression [6,7,10,11]. In our case, the neck CT showed a huge, heterogeneously enhancing thyroid mass with large central nonenhancing portion compressing the trachea, similar to previous reports. These image findings were well correlated with the pathologic findings, as the central nonenhancing area corresponds to the necrotic portion and the peripheral heterogeneously enhancing area corresponds to the viable SCC portion.
To our knowledge, no reports on imaging findings of recurred PSCCT have appeared in the English-language literature. We presume that the reason is the low prevalence and rapid progression of this disease; there is usually not an opportunity for imaging evaluation. In our case, the recurred neck mass appeared as a large, well-marginated, hypoattenuating solid mass without enhancement on contrast-enhanced CT and a large, heterogeneously hypoechoic solid mass with diffuse microcalcifications on US. Although we could not confirm the pathology of the recurred neck mass owing to the patient’s refusal, we believe that it was PSCCT recurrence on the basis of its location, the US findings comparable to the primary tumor, and the high recurrence rate of PSCCT.
When pathologic results reveal SCC of the thyroid gland, it is important to exclude metastases from other organs such as the thymus, lung, and other adjacent structures because metastatic SCC is more common than PSCCT [8]. PSCCT has a more fulminant course and poorer prognosis than metastatic SCC with a median survival of less than 6 months in the majority of cases. For the prediction of prognosis, positive immunostaining of p53 and Ki- 67 could be a poor prognostic factor, and as in our case, these are associated with poorly differentiated thyroid carcinomas and an increased risk of postoperative local recurrence [3,4,7]. Although the effect is still ambiguous, several reports suggest that radiotherapy after total thyroidectomy provides the best opportunity to control local recurrence [10,12].
In summary, PSCCT can present as a large heterogeneous hypoechoic mass with diffuse microcalcifications on US and as a well-defined heterogeneously enhancing mass with a nonenhancing necrotic portion on contrast-enhanced CT. Surgical resection or repeat biopsy should be considered if a cytologically benign thyroid mass shows imaging or clinical features of malignancy.
Notes
No potential conflict of interest relevant to this article was reported.
