Musculoskeletal ultrasound: athletic injuries of the lower extremity
Article information
Abstract
Athletic injuries of the lower extremities are commonly encountered in clinical practice. While some pathology can be diagnosed on physical exam, others are a clinical dilemma with nonspecific symptomatology. In these situations, ultrasound imaging can be utilized as an exceptional diagnostic tool, offering unique advantages over other imaging modalities. This article will review the imaging characteristics of commonly encountered athletic injuries of the lower extremity.
Introduction
Ultrasound is emerging as the preferred imaging modality to evaluate a range of lower extremity athletic injuries. In addition to high spatial resolution, an ultrasound evaluation provides several advantages allowing one to perform a dynamic evaluation tailored to the patient’s chief complaint. In this setting, the patient can provide real-time feedback, confirming symptom replication at suspected sites of abnormality. Additional information is obtained from color Doppler imaging, as well as by using graded transducer pressure to assess pain, lesion compressibility, and fluid content. Anatomic structural integrity can be tested during stress maneuvers, and pathologies present only with certain positioning or with movement can be documented. Furthermore, advances in technology are making an ultrasound evaluation increasingly less expensive and portable providing diagnostic capabilities in the clinic or even on the sidelines of sporting events [1]. However, unlike other cross-sectional imaging modalities, ultrasound is relatively operator dependent. Thus, it is extremely important that one is confident in their ability to identify frequently encountered pathology and that they have a working knowledge of relevant anatomy. This article reviews the imaging of common sports injuries involving the lower extremities.
Hip
Snapping Hip Syndrome
Snapping hip syndrome can refer to several distinct entities in which the patient feels an abnormal snapping sensation, and in some cases pain, during hip movement. Broadly, these can be divided into intra-articular and extra-articular types, where intra-articular types are the result of intra-articular bodies or prior trauma to the joint and are not well evaluated on ultrasound [2]. Extra-articular causes are well evaluated on dynamic ultrasound and are further sub-typed as external or internal by location [2,3].
Internal hip snapping, visualized at the level of the anterior inferior iliac spine with the transducer in the axial oblique plane, is the result of abnormal movement of the iliopsoas complex. At this level, the psoas major tendon is present with its muscle fibers located medially and the iliacus muscle fibers located anterolaterally (Fig. 1). As the patient flexes, abducts, and externally rotates the hip, the psoas major tendon rotates anterolaterally around the medial iliacus muscle fibers. Normally, the psoas major smoothly rotates back into position as the patient straightens the leg; however, in the snapping hip, the medial fibers of the iliacus become temporarily entrapped between the psoas major tendon and the superior pubic ramus (Fig. 1). In the later phase of leg straightening, the interposed iliacus muscle fibers suddenly move laterally and the psoas major tendon snaps against the superior pubic ramus [2-4]. Other biomechanical etiologies for symptoms at this location include a bifid psoas tendon or movement of the tendon over a paralabral cyst [2-5].

A 17-year-old girl with right snapping iliopsoas.
A. Ultrasonography in oblique transverse plane (right side of image is medial) with patient hip in frog-leg position shows medial fibers of the iliacus (arrow) temporarily trapped between the psoas major tendon (arrowhead) and superior pubic ramus (SPR). B. With leg extension, medial fibers of the iliacus move laterally (arrow), and the psoas major tendon (arrowhead) snaps down against the superior pubic ramus, returning to normal resting anatomical position. LFI, lateral fibers iliacus; FA, femoral artery.
External hip snapping occurs at the greater trochanter and is the result of abnormal movement of the iliotibial band or anterior gluteus maximus over the greater trochanter. Normally, these two structures glide smoothly over the lateral facet as the patient flexes the hip, but either may be abnormally restrained during the early phase of flexion, until it is suddenly released, springing anteriorly, and resulting in a snapping sensation (Fig. 2). Ultrasound may demonstrate a thickened iliotibial tract or band with surrounding edema [2,3].

A 20-year-old woman with right snapping iliotibial tract.
A. Transverse ultrasonography of the lateral hip (left side of image is posterior) over greater trochanter (GT) demonstrates a thickened iliotibial tract or band (arrow). B. With hip flexion, the iliotibial tract (arrow) moves anteriorly and snaps over the greater trochanter. GM, gluteus maximus.
Athletic Pubalgia
Athletes with pubic symphyseal and groin pain, termed athletic pubalgia, often play sports involving sprinting or rapid changes in direction, including soccer, football, and hockey [6]. There are multiple etiologies; however, the term "sports hernia" is a misnomer, since symptoms are often not related to a true hernia [7]. Instead, the most frequent pathology involves the common aponeurosis, which extends contiguously from each inferior rectus abdominis, over the pubis, and to the ipsilateral adductor longus [6-9].
Imaging features of athletic pubalgia include a thickened and hypoechoic common aponeurosis with or without anechoic clefts, and irregularity of the underlying pubis cortex (Fig. 3). Partial or complete tears at the adductor or rectus musculature are seen as anechoic clefts or retraction of the muscle fibers with interposed heterogeneous hematoma, respectively (Figs. 3, 4). Fluid distention of the symphysis pubis capsule may represent a symphyseal injury or osteoarthritis. These entities should also be considered in the work up of athletic pubalgia [7].
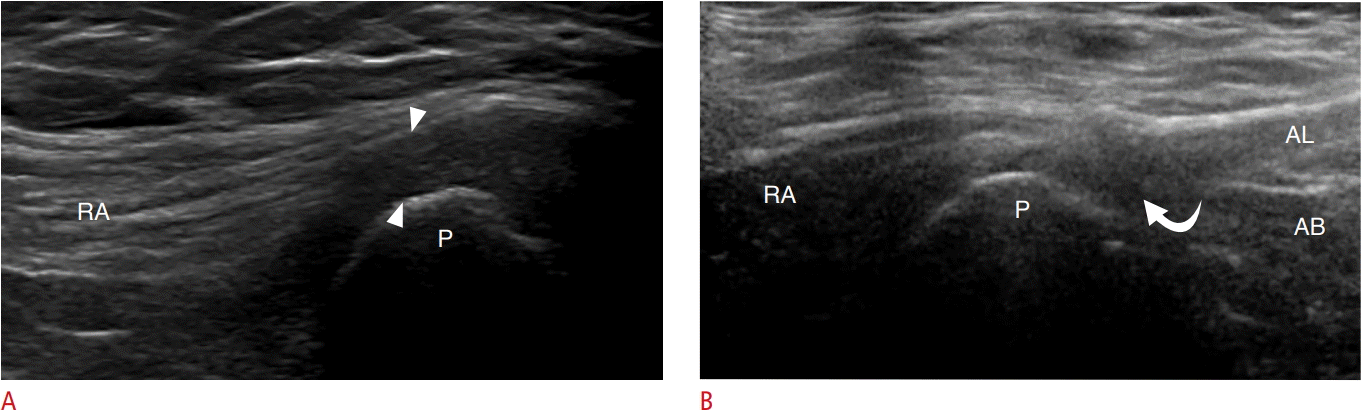
Athletic pubalgia.
A. Ultrasonography of the anterior lower abdominal wall long axis to the rectus abdominus (RA) in a 24-year-old man demonstrates a thickened, hypoechoic common aponeurosis (arrowheads) and irregularity of the underlying pubic (P) cortex. B. Similar findings are seen in a 34-year-old woman but with additional small hypoechoic clefts (curved arrow), representing partial-thickness tears at the origin of the adductor longus (AL). AB, adductor brevis.

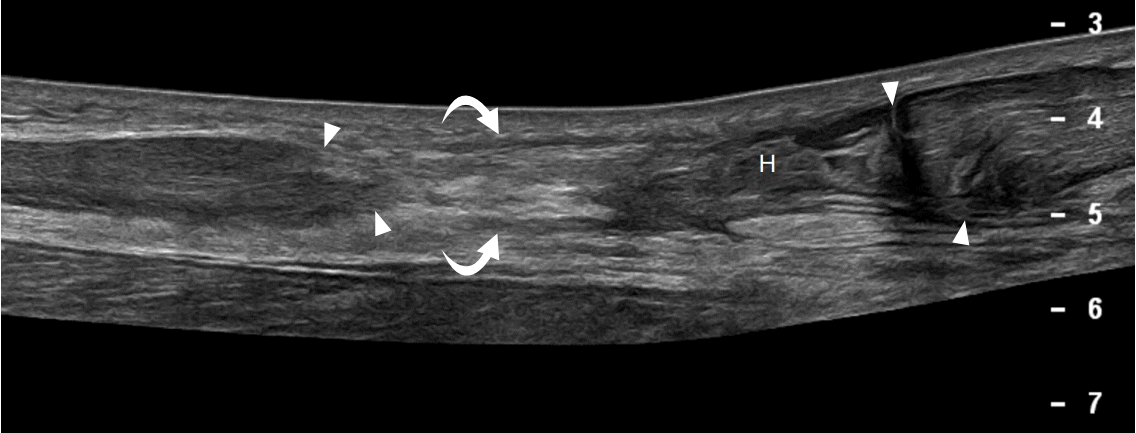
A 36-year-old man with left adductor longus full-thickness tear.
Ultrasonography of long axis to the adductor musculature demonstrates a full-thickness tear of the adductor longus tendon (AL) with heterogeneous, hypoechoic fluid representing hematoma (H) interposed between the retracted tendon (arrow) and pubic bone (P).
Rectus Femoris Injury
Abnormalities in the proximal rectus femoris are prevalent in runners and soccer players [10,11]. The rectus femoris arises as two tendons. The direct head, also known as the straight or anterior head, originates from the anterior inferior iliac spine, while the indirect head, also known as the reflected or posterior head, is located slightly laterally and posteriorly, arising along the lateral acetabular rim [12]. Both heads need to be imaged for a complete examination. To find the indirect head, begin with your transducer short axis over the direct head at the anterior inferior iliac spine, then move the transducer laterally over the acetabulum and rotate the lateral aspect of the transducer 30 degrees inferiorly to visualize the indirect head in long axis [12].
Imaging findings of proximal rectus tendon injury is similar to other tendon pathology. Hypoechoic and possibly enlarged tendon fibers representing tendinosis, anechoic clefts indicating partial tearing, and complete disruption with incongruous fibers and heterogeneous hypoechoic intervening hematoma can all be identified [11]. Calcium hydroxyapatite deposition (calcific tendinosis) may also be identified as irregular areas of hyperechogenicity with posterior acoustic shadowing [13].
Although the above-mentioned tendon pathologies are more familiar, the distinctive musculotendinous junction anatomy of the rectus femoris leads to a unique pattern of injury. The musculotendinous junction of the indirect head extends from near the hip to approximately two-thirds of the distance to the knee with the tendon located centrally. Injury of this central aponeurosis can result in edema or hematoma surrounding the tendon, resulting in a "bullseye" appearance (Fig. 5) [10,14,15]. In the young adult, anterior inferior iliac spine physeal avulsion injury should be recognized if there is focal edema, widening, or irregularity at the physis [11].
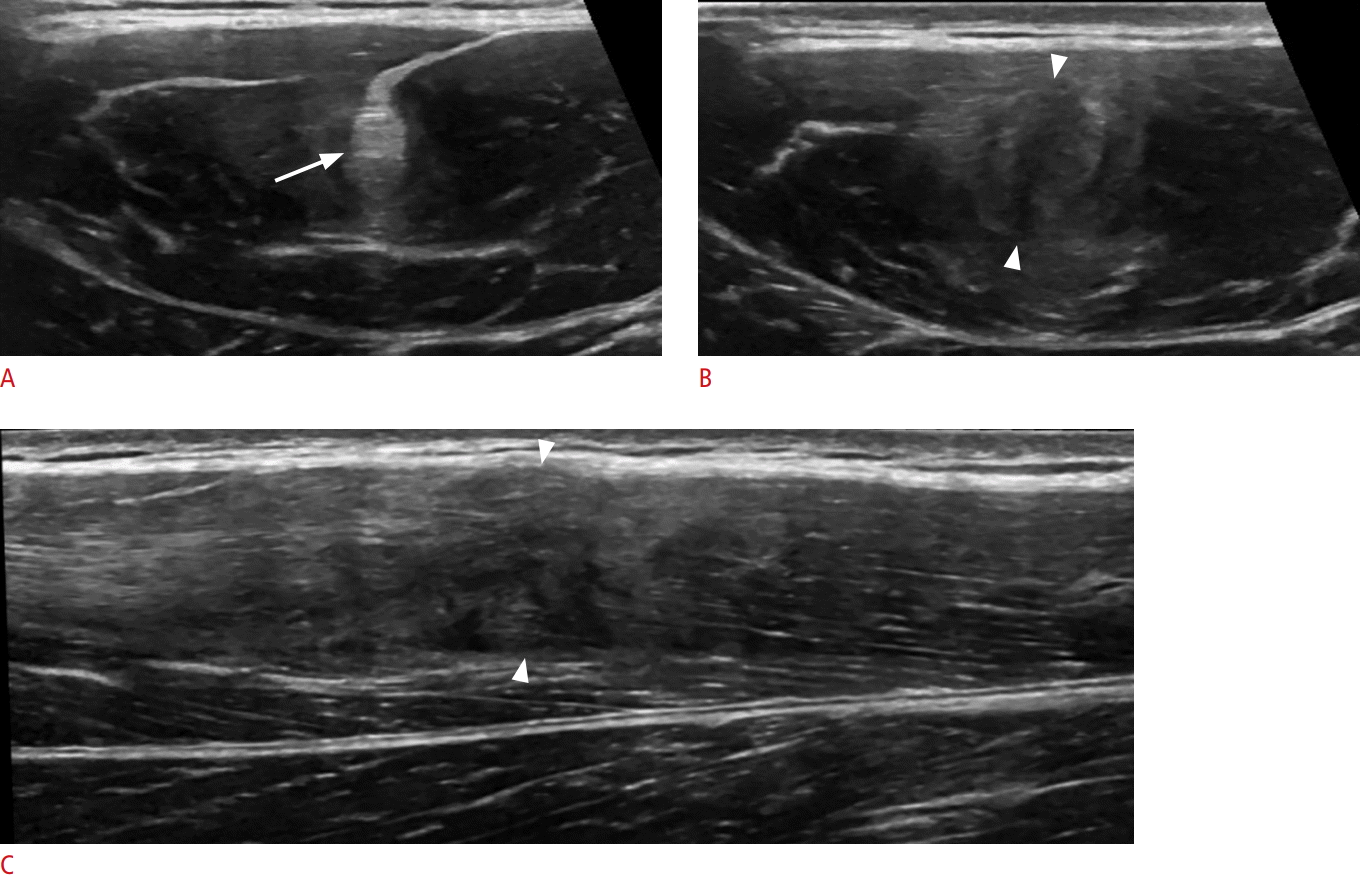
A 13-year-old boy with indirect head rectus femoris tear.
A. Proximal transverse ultrasonography demonstrates a slightly thickened, but intact rectus femoris indirect head tendon (arrow). B. Distally, the tendon is thickened, irregular, and hypoechoic (arrowheads) with the characteristic "bullseye" appearance. C. Long-axis ultrasonography demonstrates discontinuous fibers and hematoma (arrowheads) at the level of B.
Knee
Quadriceps Tendon Injury
Athletic injury to the quadriceps tendon is characteristically found in basketball or volleyball players, secondary to repeated jumping, but can also be the result of forceful flexion occurring with rapid deceleration while running [16,17]. Because the rectus femoris spans two joints, it is the most frequently injured component of the quadriceps femoris [18]. At the distal thigh, the quadriceps tendon is comprised of three layers. The superficial layer arises from the rectus femoris, the middle layer is comprised of the combined vastus medialis and vastus lateralis, and the deep layer arises from the vastus intermedius [17,19,20]. While the majority of the quadriceps tendon inserts at the anterosuperior patella, superficial rectus femoris fibers continue, attached to the anterior surface of the patella and join with the patellar tendon, referred to as the prepatellar quadriceps continuation [21].
Similar to previous descriptions, tendinosis is manifested as a hypoechoic and possibly thickened tendon (Fig. 6). While color Doppler may reveal hyperemia, the term tendinosis is used instead of tendinitis as hyperemia is due to neovascularity and not inflammation. Anechoic clefts at the patellar insertion representing tears can involve one, two, or all three layers (Fig. 7). Full thickness ruptures often occur 1-2 cm proximal to the insertion at the avascular zone; however, injury at the insertion with resultant cortical irregularity or even an echogenic, retracted and shadowing avulsion fracture may be present [19,20]. While ruptured tendon typically retracts with wavy fibers, in subtle or equivocal cases, dynamic maneuvers where the leg is passively flexed and extended, or where the patella is manually translated inferiorly, can elucidate whether proximal intact fibers move with the patella [19,20]. Separation of the prepatellar quadriceps continuation from the anterior patellar cortex with intervening hypoechoic fluid has also been described in the setting of anterior knee pain [21]. An isolated tear of the distal rectus femoris may retract and present much later after the injury as a palpable "pseudotumor" representing the retracted tendon stump (Fig. 8).

A 60-year-old woman with quadriceps tendinosis.
Ultrasonography of the anterior knee long axis to quadriceps tendon (QT) shows a thickened and hypoechoic tendon (arrowheads). Proximally, a normal fibrillar pattern is seen (arrow). P, patella; F, femur.

A 27-year-old man with partial quadriceps tear.
Ultrasonography of the anterior knee long axis to quadriceps tendon shows discontinuity and retraction of the mid deep tendon fibers (arrowheads). A linear hyperechoic fracture fragment (curved arrow) and hematoma (H) are seen. The superficial rectus femoris tendon fibers remain intact (arrows). F, femur.
Jumper’s Knee
Patellar tendinopathy resulting in anterior knee pain is colloquially referred to as "jumper’s knee." Similar to quadriceps tendon injury, and as the name would suggest, this affects individuals who play sports requiring repetitive jumping [22,23]. True tendon inflammation is not present and therefore the term tendinitis is not appropriate. Abnormal imaging findings include hypoechoic tendinosis, possible anechoic interstitial tears, and hyperemia resulting from neovascularity, which correlates with the severity of patient symptoms (Fig. 9). The deep aspect of the proximal patellar tendon is the most common site of involvement [17,22,23].

A 13-year-old boy with patellar tendinosis (jumper’s knee).
A, B. Long-axis (A) and short-axis (B) ultrasonography demonstrates a thickened, hypoechoic, and hyperemic central patellar tendon (arrowheads) at its origin with irregularity of the inferior patellar pole cortex (curved arrow). Normal fibrillar tendon is seen distally and laterally (arrow). P, patella; F, femoral condyle.
Ankle and Leg
Medial Gastrocnemius Tear
Tears of the medial head of the gastrocnemius, referred to as tennis leg, most commonly affect middle-aged athletes who note acute pain in the mid-calf during simultaneous active ankle plantar flexion and knee extension [24]. Recent studies have demonstrated that medial head of gastrocnemius tears most often occur at the distal myotendinous junction and are more common than plantaris tendon rupture, which was previously hypothesized to be responsible for this clinical syndrome [25]. In the evaluation of symptoms suspicious for tennis leg, the ultrasound operator should consider an alternative diagnosis of deep venous thrombosis, which can also present with calf symptoms.
The medial head of the gastrocnemius is best evaluated in the longitudinal orientation. Ultrasound characteristics of a gastrocnemius tear include disruption of the normal alternating linear hyperechoic and hypoechoic appearance at the distal myotendinous junction, where hypoechoic hemorrhage collection replaces the normal tapered appearance of the distal aspect (Fig. 10) [25]. Larger tears are characterized by retraction and heterogeneous fluid extending proximally, between the muscle bellies of the medial head of the gastrocnemius and the soleus. As the tear heals, fibrous tissue interposed between the medial gastrocnemius and soleus can be identified as an area of heterogeneous increased echogenicity [24,26]. Treatment for tennis leg is typically conservative.
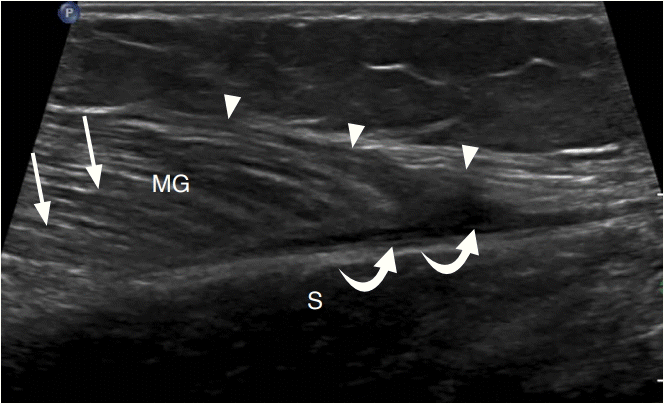
A 30-year-old woman with medial head gastrocnemius tear (tennis leg).
Ultrasonography of the calf long axis to the distal medial head of the gastrocnemius (MG) demonstrates an irregular and hypoechoic distal myotendinous junction (arrowheads) with a small hypoechoic hematoma (curved arrows) between the MG and soleus (S). Compare to the normal muscle appearance (arrows).
Plantaris Rupture
Patients presenting with a ruptured plantaris tendon describe the sudden onset of calf pain, which feels as though they have been kicked or had direct impact from a projectile, similar to patients injuring their medial head of gastrocnemius [25,27]. The plantaris muscle origin is along the posterior superior aspect of the lateral femoral condyle, with the muscle extending inferiorly and medially along the posterior knee. The long and thin plantaris tendon, located between the soleus and gastrocnemius medial head muscle bellies, terminates either on the calcaneus adjacent to the posterior medial aspect of the Achilles tendon, or on the medial Achilles tendon itself [27,28]. The plantaris may be absent in up to 7%-10% of the population [28]. On ultrasound, plantaris rupture is diagnosed by absence of the normal plantaris tendon with heterogeneously hypoechoic tubular fluid representing hematoma in its expected location between the soleus and medial head of the gastrocnemius muscle bellies (Fig. 11) [25,27,28].
Achilles Tendon Injury
Achilles tendon injury usually affects athletes in the third to sixth decades of life and results from sudden or repetitive resisted dorsiflexion [29]. Anatomically, the tendon receives contributions from the medial and lateral heads of the gastrocnemius, as well as the soleus. Though variability exists, as the tendon progresses distally, there is approximately 90° of rotation of the tendon fibers, such that the fibers from the soleus, which are deep at the proximal aspect, become medial at the insertion on the posterosuperior calcaneus [30,31]. Unlike other tendons, the Achilles lacks a tendon sheath. Instead, the Achilles tendon is surrounded by a single layer of cells referred to as a paratenon [30].
At ultrasound, the normal paratenon is identified as a thin, slightly echogenic line encapsulating the tendon. Inflammation of the highly vascular paratenon results in posterior ankle pain and is referred to as paratenonitis [30]. This can be secondary to overuse in the active patient, or secondary to inflammatory conditions such as rheumatoid arthritis or seronegative spondyloarthropathies [30]. The inflamed paratenon swells with fibroblasts and inflammatory exudate, resulting in a thickened and hypoechoic appearance on ultrasound [32]. In the acute phase, hyperemia of color Doppler and fluid between the tendon and paratenon may be present (Fig. 12) [30].
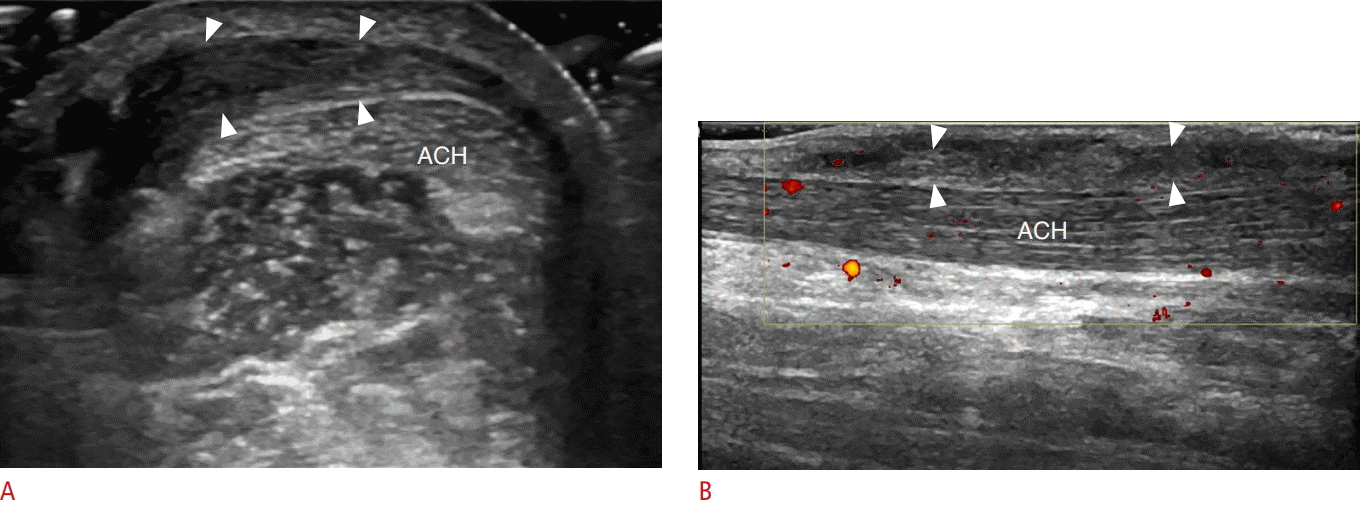
A 44-year-old woman with Achilles paratenonitis.
A, B. Ultrasonography of short axis (A) and long axis (B) to Achilles tendon (ACH) show thickened and heterogeneously hypoechoic paratenon (arrowheads).
Achilles tendon injury can occur at the myotendinous junction, the hypovascular watershed area (approximately 2 to 6 cm proximal to the insertion) commonly referred to as the "critical zone," or less commonly at the calcaneal insertion [33]. Normally the tendon is hyperechoic, fibrillar, and of uniform thickness in long axis. Tendinosis may manifest as fusiform thickening and hypoechogenicity of the tendon, with possible hyperemia from neovascularity that correlates with patient symptoms (Fig. 13) [34]. Anechoic clefts represent partial tears; thickening of the tendon to greater than 10 mm with internal heterogeneity is highly indicative of partial thickness tearing in addition to tendinosis [35]. Osseous spurring at the posterosuperior calcaneal tuberosity may be incidental or the result of insertional tendinosis. Hyperemia on color Doppler and distention of the retrocalcaneal bursa (greater than 2.5 mm) can further support this latter diagnosis [30]. Myotendinous junction injury results in disorganization of the normally parallel echogenic muscle fibers with adjacent hypoechoic edema [33].
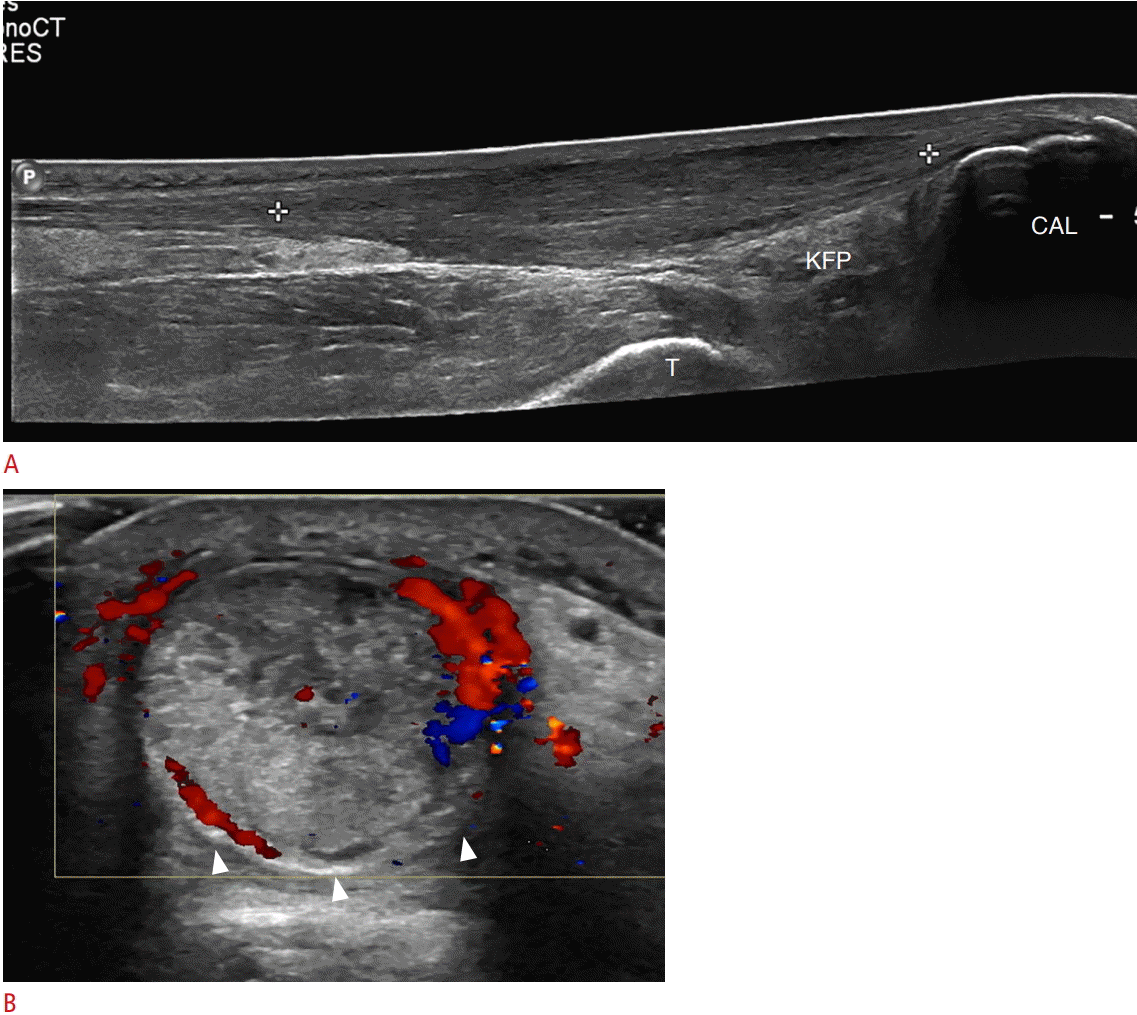
A 61-year-old woman with Achilles tendinosis.
A, B. Ultrasonography of long axis (A) and short axis (B) to Achilles tendon demonstrates a long segment of thickened tendon (between the calipers). This is the watershed zone approximately 2-6 cm proximal to the insertion on the posterior calcaneus (CAL). Hyperemia representing paratenonitis (arrowhead) is also noted. T, tibia; KFP, Kager fat pad.
With a full-thickness complete Achilles tendon tear, there retraction of the proximal tendon fibers and a positive Thompson test, where squeezing the calf does not result in the normal plantarflexion of the foot [36]. On ultrasound, retracted tendon fibers are wavy and irregular with heterogeneously hypoechoic intervening fluid. It is important to document both the stump quality as well as the residual gap between the tendon stumps during dorsiflexion, as this helps guide clinical decision-making regarding surgical versus non-surgical management (Fig. 14). Full-thickness tears present as tendon discontinuity and retraction during dynamic evaluation with passive plantar flexion and dorsiflexion [30]. The use of dynamic imaging that demonstrates tendon discontinuity is an important component of the ultrasound examination to achieve high accuracy in full-thickness tear diagnosis. The plantaris almost uniformly remains intact and should not be misinterpreted as intact Achilles tendon fibers.
Peroneal Tendinopathy
A frequent cause of posterolateral ankle pain, the peroneal tendons are prone to overuse injury [37]. While the peroneus longus, arising proximally from the fibula and tibia, has its myotendinous junction well before it reaches the ankle, the peroneus brevis muscle, originating from the distal fibula, tapers distally to the lateral malleolus where the tendons pass posteriorly in the retromalleolar groove. The peroneus brevis is typically in contact with the fibula, between the bone and the peroneus longus, which likely accounts for its predisposition to injury at this location. The tendons are held in place as they pass behind and distal to the lateral malleolus by the superior and inferior peroneal retinacula. Distally, the tendons course anteriorly, on either side of a bony excrescence from the calcaneus, termed the peroneal tubercle, until the brevis inserts at the base of the fifth metatarsal and the longus, having curved along the plantar aspect of the foot, inserts at the base of the first metatarsal and medial cuneiform [37,38].
At ultrasound, peroneal tendinosis appears as hypoechogenicity with possible tendon enlargement. Anechoic clefts indicate superimposed interstitial tearing. An anechoic cleft that extends to the surface of the tendon, commonly the peroneus brevis, is termed a longitudinal split tear (Fig. 15). A full-thickness complete tear presents as tendon discontinuity with retraction. Tenosynovitis can vary from anechoic fluid distention of the tendon sheath to mixed-echogenicity but predominantly hypoechoic hypervascular synovitis [37,38].

A 59-year-old woman with peroneus brevis longitudinal split tear.
Ultrasonography of short axis to peroneus longus tendon (PL) at the level of the distal fibula (F) demonstrates a longitudinal split tear of the peroneus brevis with two separate bundles (arrowheads) with hypoechoic tenosynovitis (curved arrows).
Peroneal Tendon Subluxation and Dislocation
Abnormal movement of the peroneal tendons may result in snapping, pain, and tendon injury, and is best evaluated with ultrasound during dynamic imaging with ankle dorsiflexion and eversion [39,40]. Peroneal tendon subluxation and dislocation are the sequelae of injury to the superior peroneal retinaculum, which normally holds the peroneal tendons in place along the posterior fibula in the retromalleolar groove. When injured or torn, the retinaculum may appear hypoechoic or discontinuous, with or without an avulsion fragment retracted from its insertion on the fibula [40]. During dorsiflexion and eversion stress maneuvers, one or both peroneal tendons may partially move anterior and lateral from their normal location (subluxation) or completely displace (dislocation), and return during rest, predisposing to tenosynovitis and tendon tear (Fig. 16) [39].

A 57-year-old woman with peroneal tendon subluxation/dislocation.
A, B. Transverse ultrasonography at the distal fibula (F) at rest (A) and during dorsiflexion (B) and eversion demonstrate an abnormally thickened superior peroneal retinaculum (arrowheads) which is stripped from the fibula. This allows anterior dislocation of the peroneus longus (PL) and subluxation of the peroneus brevis (PB) in B.
Intrasheath peroneal tendon subluxation occurs when the peroneus longus and brevis tendons abnormally alternate positions between themselves producing a snapping sensation, while remaining deep to an intact superior retinaculum. This condition may be associated with a longitudinal split tear of the peroneus brevis, where the tendon of the peroneus longus moves in between the two brevis tendon bundles during stress. Intrasheath subluxation is associated with an abnormally convex posterior border of the ateral malleolus, a low lying brevis muscle where the myotendinous junction extends distal to the lateral malleolus, or an accessory peroneus quartus muscle and tendon at this level [40].
Anterior Talofibular Ligament Tear
One of the most biomechanically important ligaments of the ankle, the anterior talofibular ligament (ATFL) is also the most frequently and often first to be injured, and is the result of abnormal inversion stress [41]. In order to identify the ligament, either palpate or locate the extreme distal tip of the fibula with ultrasound in the transverse plane. Then, slide the transducer anteriorly and slightly superiorly until both the fibula and talus are in the field of view. The obliquely oriented ATFL may be slightly hypoechoic from anisotropy, but altering the probe angle with a heel-toe maneuver should reveal the normal echogenic, fibrillar pattern [42].
Injuries to the ATFL produce imaging features similar to other ligamentous injuries. In an acute partial tear, the ligament is hypoechoic with some intact fibers remaining. In an acute full thickness tear, the fibers are discontinuous or absent, replaced by heterogeneous hematoma (Fig. 17). Avulsion fractures are identified as echogenic foci adjacent to sites of ligamentous insertion. Dynamic imaging with an anterior drawer test can help distinguish between a partial or full thickness tear; talus motion can assist in visualizing intact ligament fibers, and asymmetric anterior talus subluxation suggests ligament failure. This maneuver is completed with the patient prone, the transducer placed over the ATFL, and stress placed on the posterior calcaneus [41]. With a chronic tear, the ligament may be absent, thinned, or thickened, but the patient should not have associated symptoms with transducer pressure [43]. With suspected lateral ankle ligament injury, the calcaneofibular ligament should also be assessed, which typically tears in succession after the ATFL tears, appearing as a hypoechoic and thickened ligament adjacent to the calcaneal body (Fig. 18).

A 17-year-old girl with acute anterior talofibular ligament tear.
Ultrasonography over the anterolateral ankle in the transverse plane demonstrates complete disruption of the anterior talofibular ligament with irregular and frayed tibial and fibular stumps (arrowheads) and interposed heterogeneous hypoechoic hematoma (H). T, talus; F, fibula.
High Ankle Sprain
During extreme ankle eversion, the first of the ankle syndesmotic ligaments to be injured is the anterior inferior tibiofibular ligament (AiTFL). This ligament is identified by first orienting the transducer over the anterior talofibular ligament, then rotating the talar (medial) side of the probe superiorly so that the probe is oblique and the fibrillar ligament comes into the field of view. The resulting imaging plane should be similar to that for the calcaneofibular ligament but on the tibial side of the fibula. Ligament tears will appear as hypoechoic thickening or ligament discontinuity (Fig. 19) [44]. Because the AiTFL is the first ligament to be injured in eversion, if this is intact, interosseous membrane injury is uncommon [45].

A 49-year-old woman with acute anterior inferior tibiofibular ligament tear.
Ultrasonography in the transverse oblique plane at the level of the distal tibia demonstrates complete disruption of the anterior tibiofibular ligament with irregularity of the remaining stumps (arrowheads) and heterogeneous, hypoechoic intervening fluid (H). T, tibia; F, fibula.
If the AiTFL is injured, however, injury to the interosseous membrane, commonly referred to as a "high ankle sprain," should be excluded as this may lead to delayed healing and recovery, instability, and accelerated degenerative change [46]. The interosseous membrane is identified as a thin echogenic linear structure between the tibia and fibula and may be discontinuous, thickened, and hypoechoic when injured. Dynamic imaging can be a helpful tool in evaluating integrity, assessed by measuring the tibiofibular clear space 1 cm proximal to the joint line. Normally there should be minimal difference in the measurements of the tibiofibular clear space between internal and external rotation at the ankle (usually a difference of less than 2 mm, but up to 5 mm). Significant widening of the clear space during external rotation indicates injury to the interosseous membrane. Injuries may propagate proximally through the interosseous membrane and exit through the proximal fibula, resulting in a Maisonneuve fracture, by definition, which can be identified as a cortical step off [44,45]. In the setting of clinical suspicion, radiographs of the proximal fibula are often helpful for confirmation of a Maisonneuve fracture.
Muscle Hernia
Muscle herniations often present a diagnostic dilemma, as patients may be referred for pain, cosmetic mass, or concern for a tumor. A muscle hernia occurs when normal muscle protrudes from its normal anatomic compartment through a defect in the overlying fascia and into the subcutaneous fat. Eighty-nine percent of muscle hernias occur in the lower extremities, most frequently involving the tibialis anterior muscle [47], but also often involving the peroneal, gastrocnemius, soleus, quadriceps and erector spinae muscles [47,48]. Pain associated with this entity may be related to transient muscle strangulation or superficial nerve entrapment; however some have described a correlation with chronic exertional compartment syndrome, which may serve an alternative etiology for pain [47]. Treatment depends on symptomatology and may range from reassurance that the lesion is benign, to activity modification, compression stockings and non-steroidal anti-inflammatory medications to surgical intervention with fasciotomy [47].
During ultrasound evaluation, a few technical considerations can improve diagnostic accuracy. The mass should be palpated by hand and marked with a pen, as the abnormality may be difficult to palpate with the ultrasound probe; liberal use of gel as a stand-off enables the use of light pressure ensuring that the hernia is not reduced or obscured, and dynamic evaluation during muscle contraction or with the patient standing can accentuate the hernia [49].
Normal fascia should appear as a thin echogenic line immediately superficial to the muscle. A muscle hernia is diagnosed when imaging demonstrates a defect in the echogenic fascia with a portion of the underlying muscle protruding through and over the fascia, often assuming a mushroom shape (Fig. 20). The herniated muscle may be slightly less echogenic than adjacent normal muscle and may be secondary to anisotropy or atrophy [47,48]. Blood vessels are sometimes noted traversing the fascia at the site of the defect and have been hypothesized as potential cause of focal fascial weakness [49].
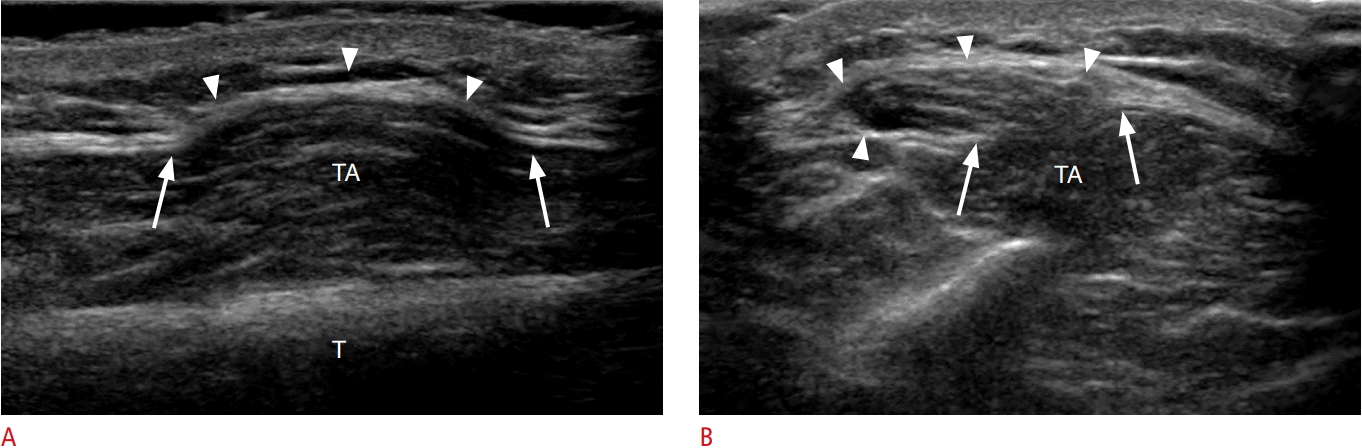
A 21-year-old man with tibialis anterior muscle hernia.
A, B. Longitudinal (A) and transverse (B) ultrasonography over the anterior leg demonstrates a defect in the deep fascia (arrows) overlying the tibialis anterior (TA). Muscle fibers are seen herniating through the defect into the overlying subcutaneus fat (arrowheads). T, tibia.
Foot
Plantar Fasciopathy
Distance running may result in chronic and sometimes exquisite heel pain. One etiology for this type of pain is repetitive microtrauma to the plantar aponeurosis, resulting in plantar fasciopathy [50]. Inflammation is absent in this condition therefore the term fasciitis should not be used. Originating from the calcaneal tuberosity, the plantar aponeurosis is a thick fibrous band that is divided into three cords. The central cord is the thickest and most often injured, enveloping the flexor digitorum brevis muscle and dividing anteriorly into five fascicles that insert distally onto the skin, flexor tendon sheaths, and plantar plates of the phalanges [51]. The medial and lateral cords envelop the abductor hallucis brevis and abductor digiti minimi, respectively, and are rarely injured.
Ultrasound features of plantar fasciopathy include a thickened (greater than 4 mm) and hypoechoic aponeurosis close to its calcaneal attachment (Fig. 21) [51]. Anechoic clefts may be seen in interstitial tearing, and, more rarely, complete tears demonstrate retraction of the discontinuous aponeurotic fibers with intervening heterogeneous hematoma. Hyperemia may be present on color Doppler evaluation and the patient often exhibits focal tenderness over the abnormality. A bony enthesopathic spur at the calcaneal tuberosity may be present in symptomatic or asymptomatic individuals [50,51]. Contrary to popular belief, recent studies have demonstrated no correlation between fascial thickness and degree of symptoms. Biconcavity, however, is a feature that has been associated with a diminished response to supportive therapy [52].
Turf Toe
Turf toe is an injury at the first metatarsophalangeal (MTP) joint that results from an axial load while the joint is in hyperextension. This typically affects American football players who play on hard, artificial surfaces with flexible shoes, resulting in injury to the supporting structures of the joint [53]. The anatomy of the first MTP capsuloligamentous complex consists of a cartilaginous plantar plate, two sesamoid bones, several ligaments (intersesamoidal, metatarsosesamoidal, sesamoid phalangeal, collateral), and a joint capsule, with support provided by the tendons of the flexor hallucis brevis and longus, and abductor and adductor hallucis [53,54]. The plantar plate originates at the metatarsal neck and inserts at the plantar aspect of the base of the proximal phalanx associated with the joint capsule [53,54].
Ultrasound in the setting of turf toe may demonstrate hypoechoic soft tissue edema, a thickened and hypoechoic plantar plate, ligament thickening or discontinuity, a hypoechoic cleft between the plantar plate and proximal phalanx, or an echogenic avulsion fracture from the plantar plate insertion at the base of the proximal phalanx (Fig. 22). Although the sensitivity of ultrasound in the diagnosis of plantar plate tear is 96%, a heterogeneous appearance may be seen as an asymptomatic finding, which likely accounts for its poor specificity [55]. On physical exam there may be ecchymosis at the volar MTP joint and focal pain. A drawer test, where the MTP is stressed dorsally, is positive when there is excessive dorsal translation of the proximal phalanx and supports the diagnosis of turf toe [53,54,56].
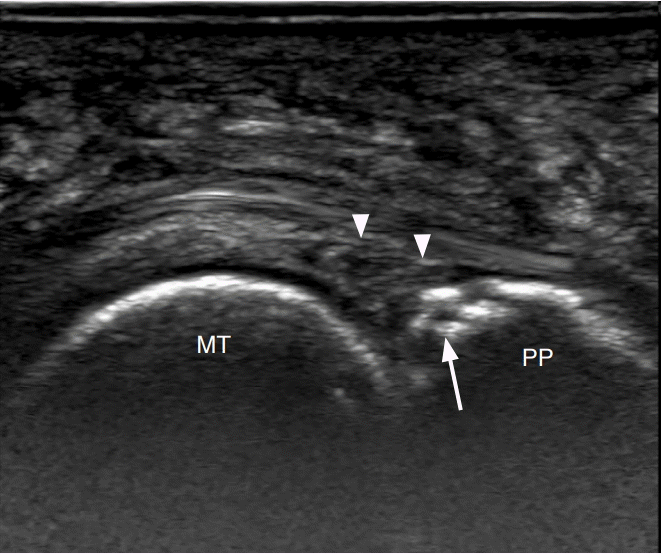
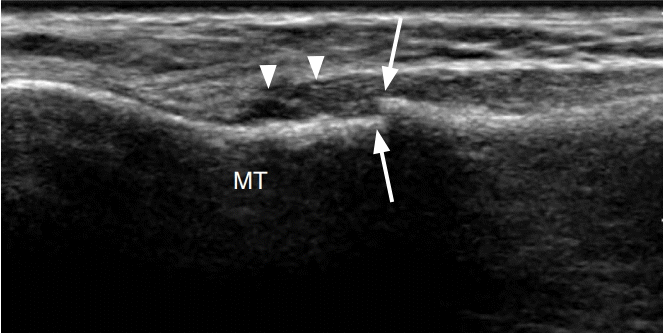
A 57-year-old woman with plantar plate injury.
Longitudinal ultrasonography at the plantar aspect of the second metatarsophalangeal joint demonstrates abnormal heterogeneous and hypoechoic appearance of the plantar plate (arrowheads) with cortical irregularity at its insertion on the proximal phalanx (arrow). MT, metatarsal; PP, proximal phalanx.
Stress Injury and Fracture
Fatigue-type stress injury, resulting from excessive stress on otherwise healthy bone, most often affects the second and third metatarsal shafts in a ballet dancer, running athlete, or military personnel, but can involve any metatarsal [53,57]. Often the history will reveal a sudden recent increase in activity level. Manifesting as vague forefoot pain during exercise, stress injury follows a predictable pattern, as repetitive mechanical forces exceed the ability of the bone to grow and repair itself, resulting in microfractures [57]. Periostitis and periosteal new bone formation are seen in the least severe cases, followed by bone marrow edema, then incomplete fracturing and eventually a completed fracture.
Although it is not feasible to evaluate all of the osseous structures on ultrasound, a targeted evaluation at the patient’s area of point tenderness may reveal findings supportive of this diagnosis. Hypoechoic soft tissues indicating swelling, cortical irregularity, and periosteal thickening may be seen at the site of stress injury, while a frank cortical step-off is diagnostic of a fracture (Fig. 23) [58,59].
Conclusion
As clinical applications for ultrasound in musculoskeletal care continue to expand, it has become increasingly important to have an understanding of common athletic injuries in the lower extremities and their sonographic appearances. This article highlights several such injuries in an effort to review basic anatomic relationships, pathologic appearances, and the importance of the dynamic ultrasound evaluation.
Notes
No potential conflict of interest relevant to this article was reported.