Intralesional saline injection for effective ultrasound-guided aspiration of benign viscous cystic thyroid nodules
Article information
Abstract
Purpose:
We aimed to evaluate the efficacy and safety of vigorous saline injection for viscous cystic thyroid nodules.
Methods:
Eighteen patients who underwent ultrasound-guided aspiration for viscous cystic thyroid nodules using a saline injection were included in our study. After failing to aspirate the cyst by the usual method, we vigorously injected saline into the cyst in multiple directions to break up and liquefy the viscous cystic contents to enable aspiration. The initial and the residual volume of the nodule were calculated, and the volume reduction rate and the time taken to perform the aspiration were recorded.
Results:
The mean volume of the cystic nodules before aspiration was 11.0 mL (range, 1.2 to 26.0 mL), while the postaspiration volume was 4.2 mL (range, 0.2 to 14.5 mL). The mean aspirated volume was 63.7% of the initial volume. The mean procedure time was 12.4 minutes (range, 5 to 26 minutes). There were no significant complications related to the procedure.
Conclusion:
A vigorous saline injection followed by aspiration can be a useful method to aspirate viscous cystic thyroid nodules as a prestep for further intervention or simple management.
Introduction
Benign nodular thyroid disease is common among adults, and its prevalence increases with age. In ultrasound (US), 15%-25% of solitary thyroid nodules are found to be cystic or predominantly cystic [1]. Because the simple aspiration of cystic thyroid nodules can reduce pressure-related symptoms and cosmetic problems, despite a tendency toward recurrence, US-guided fine-needle aspiration (US-FNA) is frequently used for the diagnosis and treatment of symptomatic cystic thyroid nodules.
Several management modalities for symptomatic cystic thyroid nodules include simple aspiration, ethanol injection, radiofrequency ablation, and surgery [2-4]. However, the most simple and cost-effective first-line method is simple aspiration [5], which facilitates the effectiveness of other nonsurgical treatment methods in the lacunae evacuated viscous materials [6]. However, simple cyst aspiration is sometimes difficult or impossible in viscous cystic thyroid nodules. These nodules are defined as nodules that cannot be aspirated, even with an 18-gauge needle, due to the high viscosity of their contents, and they may account for approximately 30% of all cystic thyroid nodules [7].
Some studies on the evacuation of viscous cystic thyroid nodules have used repeated ethanol injection or a large pigtail catheter connected to a suction pump [8-10]. These procedures are relatively more invasive and need special equipment, which is very expensive. To overcome these problems, we tried a new way to aspirate viscous cystic fluid in a safe and cost-effective manner. If we inject viscous cystic nodules with saline, the viscosity of these nodules decreases and subsequent aspiration becomes easier. Therefore, in this study, we aimed to evaluate the efficacy and safety of vigorous saline injection for viscous cystic thyroid nodules.
Materials and Methods
Patients
Our institutional review board approved this retrospective study and required neither patient approval nor informed consent. However, informed consent was obtained from all patients for US-FNA prior to each procedure.
From December 2009 to June 2011, 18 patients who underwent US-FNA for viscous cystic thyroid nodules using a vigorous saline injection following aspiration were included in our study. A viscous cystic thyroid nodule was defined as follows: the cystic component was more than 50% of the total nodule volume, which was less than 0.5 mL of the viscous cystic content aspirated with an 18-gauge needle connected to a 10-mL syringe, despite the use of an aspirator [10]. Cyst aspiration was mainly performed in these patients due to cosmetic/pressure symptoms (n=16), or at the patient’s request due to a fear of cancer (n=2). The benign cytological results of the cystic fluid and cyst wall were obtained with previous US-FNA.
Procedure
Aspiration was performed by two board-certified radiologists with 10 and 15 years of experience in thyroid imaging. The patient was in a supine position with mild neck extension. We used a 10-MHz linear probe on a real-time ultrasound system (iU22; Philips Medical Systems, Bothell, WA, USA). The volume of the nodule before aspiration was calculated using the following equation: V = Πabc/6, where V denotes the volume, a represents the largest diameter, and b and c denote the other two perpendicular diameters.
After sterilizing the skin with 70% ethanol, we inserted cystic nodules with an 18-gauge centesis needle via the transisthmic approach [11] and vigorously injected normal saline (Fig. 1). Immediately after this step, the viscous materials were easily extracted by applying a mild negative pressure in the 10-mL syringe. The procedure was terminated when the cystic nodules were almost completely collapsed. The residual volume of the nodules was calculated, and the time taken to perform the aspiration was recorded. Subsequent percutaneous ethanol injection (PEI) for 11 cases was performed. In this study, indications for ethanol injection were not assessed because our study focused on a preparatory procedure for aspiration. However, in our institution, ethanol injection is usually performed in recurrent symptomatic thyroid benign cysts. After the maximum volume of the internal fluid was aspirated using a vigorous saline injection following the aspiration, 99% ethanol was injected slowly into the cystic space. The volume of the injected ethanol usually depends on the nodule size. However, the maximal volume of ethanol was less than 10 mL in all cases. The injected ethanol left and was absorbed without any problems. Patients were observed for at least 30 minutes after the procedure.
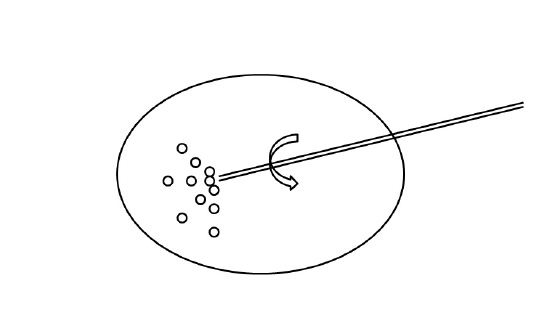
Schematic representation of how to effectively aspirate a viscous cystic thyroid nodule after saline injection.
The keys to this procedure are that the pressure applied to inject normal saline should be as strong as possible (in order to break up the gelatinous material) and that the needle should be rotated and stirred multidirectionally after saline instillation.
Follow-up US and clinical examinations were reviewed. We measured the residual volume of the lesions on each examination and calculated the volume reduction rate. The Mann-Whitney U test was used to compare the volume of the cysts and the volume reduction rates according to the PEI.
Results
The results of our 18 patients are summarized in Table 1. The mean volume of the cystic nodules before aspiration was 11.0 mL (range, 1.2 to 26.0 mL), while the postaspiration volume was 4.2 mL (range, 0.2 to 14.5 mL). The mean amount of the injected saline was 8.94 mL (range, 2 to 32 mL), while the mean volume of the aspirated thick internal content was 14.4 mL (range, 3 to 39 mL). The mean aspirated volume was 63.7% of the initial volume (Fig. 2). The mean procedure time was 12.4 minutes (range, 5 to 26 minutes). Eleven of the 18 patients subsequently underwent percutaneous ethanol ablation, and the remaining seven patients did not opt for further intervention. There were no significant complications or discomfort related to this procedure. The mean follow-up period was 9 months (range, 6 to 28 months). The mean volume reduction rates between the subsequent PEI group and no PEI group after aspiration are summarized in Table 2. The mean volume reduction rate was higher in patients treated with further PEI. However, there was no statistically significant difference in the last volume and the volume reduction rates between the two groups (P=0.571 and 0.643, respectively). There was one patient (case number 17) with recurrence 6 months after the vigorous saline injection with aspiration.
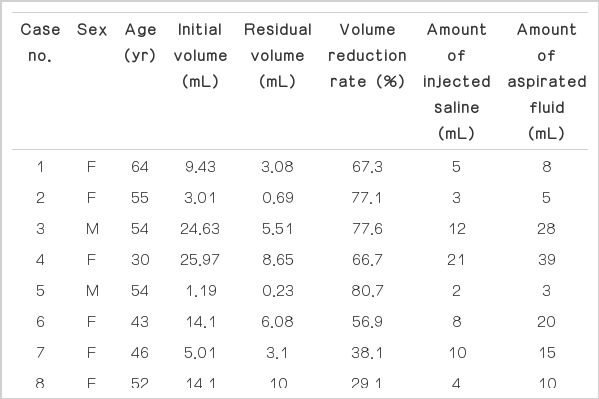
Clinical results of simple aspiration using saline injection in 18 patients with viscous cystic thyroid nodules
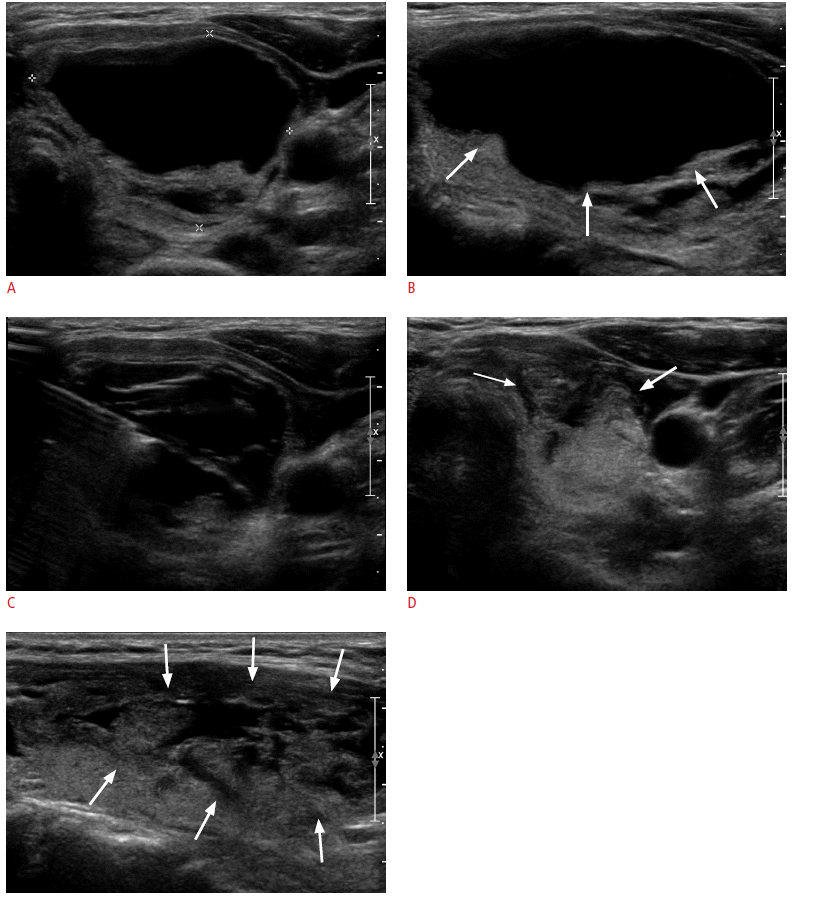
A 30-year-old woman with neck discomfort.
Ultrasonograms (A, transverse; B, longitudinal view) show a 26-mL (maximum diameter, 5.3 cm) cystic nodule with septation in the left thyroid, which could not be aspirated because of the high viscosity of the internal content (arrows). After saline injection, viscous cystic materials were easily evacuated with a long centesis needle via a transisthmic approach (C). After the aspiration, the cystic component nearly collapsed, and an 8.6-mL residual solid portion (arrows) remained on ultrasonograms (D, transverse; E, longitudinal view).
Discussion
Large cysts sometimes cause cosmetic problems or pressure symptoms but rarely accompany malignant neoplasia, particularly for pure cysts [12]. Therefore, attempting simple aspiration for the initial diagnosis and treatment of thyroid cystic nodules is reasonable in spite of the fact that the recurrence rates are as high as 80%, depending on the initial volume and the internal contents of the nodules, and the number of aspirations [13].
A particular type of cystic thyroid nodules, viscous cystic nodules, has only been addressed in a few publications. In 1974, Miller et al. [14] described a series of 68 thyroid nodules among which they observed nodules that were “entirely cystic but their gelatinous contents apparently would not pass through the 18-gauge needle.” Until the late 1990s, only surgery was used to treat viscous cystic nodules. In 1996, Zingrillo et al. [9] and Cho et al. [8] reported an attempt to treat a high-density viscous nodule with a two-stage ethanol ablation or repeat instillation of ethanol. However, highly viscous fluid is difficult to aspirate and inhibits the diffusion of ethanol. Therefore, ethanol ablation was less effective in gelatinous thick fluid than in pure serous cystic fluid [6,8]. Moreover, because these previous methods have problems, such as the large number of treatment sessions required and the delayed completion of treatment, Sung et al. [10] tried a one-step ethanol ablation technique in which the thick gelatinous contents were aspirated through large-bore needles or a catheter connected to a suction pump. However, this method is relatively aggressive and requires special equipment that is not always readily available in the US room. Despite their good results, their methods in practice are not convenient and incur additional costs. As demonstrated by our results, our saline injection method is easy to perform and effective in evacuating internal gelatinous contents.
In a randomized double-blind controlled study conducted by Bennedbaek and Hegedus [15], patients were divided into two groups: US-guided subtotal cyst aspiration, instillation and flushing with 99% ethanol or with isotonic saline, and subsequent complete fluid aspiration without removing the needle after 2 minutes. The effect on the recurrence rate of benign recurrent thyroid cysts between ethanol and isotonic saline for the subsequent complete emptying was evaluated. In terms of the number of treatment sessions and the recurrence rate, they concluded that the treatment of recurrent thyroid cysts with ethanol is superior to simple aspiration and flushing with saline. Moreover, the former method was devoid of serious side effects. However, drawbacks of ethanol ablation exist, and this treatment is less effective for viscous cystic thyroid nodules than for purely serous cystic thyroid nodules because the effect of ethanol ablation depends on the amount of aspirated fluid and its even diffusion inside the tissue [6]. Therefore, near-total aspiration of viscous cysts is required to achieve successful ethanol ablation. However, in their study, they did not comment on the number of viscous cystic lesions included or the method of subtotal cyst aspiration in the cases of viscous cysts. Therefore, to the best of our knowledge, this is the first report on the use of saline for effective aspiration of viscous cysts, either as a preparatory step or as a definitive treatment itself.
The keys to our procedure are that the pressure applied to inject saline should be as strong as possible (in order to break up the gelatinous materials) and that the needle should be rotated and stirred multi-directionally during saline instillation. By performing these steps, we ensure that the decrease in viscosity is homogeneous across the lesion. When a needle is inserted into a nodule, the transisthmic approach makes it safer and easier to handle a needle than the vertical approach [11]. Finally, we can liquefy the jellylike cystic contents and achieve aspiration of a remarkably large volume of viscous material as compared to when this method is not used, as in previous reports. After this step, we may perform additional ethanol ablation or finish the procedure. In our study, 11 patients received further ethanol ablation. The advantage of this method is that it is simple, cost-effective, and less time-consuming. Although most cases could be treated within 20 minutes, in one case (case number 7), it took us 26 minutes to aspirate a cyst. This was the first case in which the novel method we describe was used, and accounts for the time we initially spent attempting to use the standard method of aspiration without saline. Because it was too difficult to aspirate the viscous cystic fluid, we tried to inject some saline into the cyst and found that it was easier to aspirate fluid when a saline injection is used, thus discovering our new technique. We always plan to manage thyroid cysts with an initial simple aspiration as the first treatment choice for symptomatic cystic nodules. Additional treatment such as ethanol injection is indicated when, despite performing a simple aspiration, cystic lesions are recurrent or persistent with symptoms. The greater the cystic portion of the treated nodules, the more dramatic the reduction when using this method. That is, the volume of the solid portion of the cysts affects the residual volume after treatment.
This study could not be compared with a control because the procedure carried out in this study was subsequently performed only when the routine aspiration of the control group failed.
In conclusion, our experience shows that saline injection into cysts can lead to the effective aspiration of viscous cystic thyroid nodules because the saline decreases the viscosity of these nodules.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This study was supported in part by the Research Fund of the Korean Society of Ultrasound in Medicine.

