A pure mucocele-like lesion of the breast diagnosed on ultrasonography-guided core-needle biopsy: is imaging follow-up sufficient?
Article information
Abstract
Purpose:
To evaluate the upgrade rate of ultrasonography (US)-guided core-needle biopsy (CNB) of the breast for a pure mucocele-like lesion (MLL), to evaluate the clinical and radiologic features, and to correlate the image-pathologic features further on to guide the management of MLL.
Methods:
Between January 2003 and February 2013, 14-gauge US-guided CNB was performed in 18,111 cases. Thirty-two cases associated with MLL were identified, and five cases of MLLs associated with breast carcinoma or with other high-risk breast lesions (i.e., atypical ductal hyperplasia [ADH], papillary lesions, lobular carcinoma in situ, and radial scar complex) were excluded. Among these 27 pure MLLs, 21 cases with surgical or vacuum-assisted excision (VAE) pathology were included in our study. Medical records, mammograms, and ultrasonograms were reviewed for the clinical and radiologic features of the cases.
Results:
Among the 21 cases with pure MLLs at CNB, the final pathology showed a 0% proportion of cases upgraded to malignancy. All the 21 cases with either surgical or VAE pathology were benign MLLs including three cases of focal involvement of ADH (14.3%). The common features were mammographic features of microcalcifications that were round in shape and had a grouped distribution. The US features included oval shape, circumscribed margin, parallel orientation, complex solid and cystic echo pattern, no posterior feature, and complex solid and cystic echoic masses. The predominant Breast Imaging Reporting and Data System (BIRADS) category was 4A. All the lesions showed image-pathologic concordance.
Conclusion:
For pure MLL on US-guided CNB with image-pathologic concordance, close imaging follow-up might be considered instead of surgical excision.
Introduction
Mucocele-like lesion (MLL) of the breast was originally described as a benign lesion composed of multiple cysts lined by cytologically uniform flat or cuboidal to columnar epithelium with extravasated mucin [1]. Later reports have shown an association of MLL with columnar cell lesions (CCLs), atypical ductal hyperplasia (ADH), ductal carcinoma in situ (DCIS), and mucinous carcinoma, and have suggested that MLL and mucinous carcinoma may represent the two ends of the pathological spectrum of the mucinous lesions of the breast [2].
Despite the well-known reliability of core-needle biopsy (CNB) as a diagnostic method for breast lesions, there are conflicting reports about the sufficiency of CNB to diagnose benign MLLs; therefore, due to the limited material available on CNB specimen, paucicellular mucinous carcinoma cannot be excluded [3-8]. Owing to the risk of associated malignancy, excisional biopsy is suggested after the identification of MLL on CNB, particularly when the mass is palpable or radiologically evident, or when the sample exhibits ADH [6,7]. However, for pure MLL on CNB, that is, MLL without any associations with CCL, ADH, DCIS, or invasive cancer, some recent reports have suggested that excision is unnecessary [1,9]. Still, there are limited studies regarding the clinical and radiologic features that can help to predict malignancy or the image-pathologic correlation of pure MLLs diagnosed on CNB to determine which lesions truly require or do not require excision. The purpose of this study was to evaluate the outcome and the clinical and radiologic features of pure MLLs diagnosed on CNB, and to further correlate their imagepathologic features in order to guide their appropriate management.
Materials and Methods
Patients
This retrospective review of images and medical records was approved by the Institutional Review Board of our institution, and the requirement for informed patient consent was waived. We retrospectively reviewed the pathology results dated from January 2003 to February 2013 in the CNB database files at our institution. Out of 18,111 cases of ultrasonography (US)-guided CNB, 32 cases associated with MLLs were identified. All the MLLs associated with breast carcinoma (i.e., DCIS and invasive cancer) or with other highrisk breast lesions (such as ADH, papillary lesions, lobular carcinoma in situ , CCL, and radial scar complex) were excluded from this study, and 27 cases of pure MLL were identified. Among these 27 cases, six cases were also excluded because there was neither surgical nor vacuum-assisted excision (VAE) pathology. Among these cases, there were 19 cases with surgical pathology and two cases with VAE pathology. The collected data of each patient included the patient’s medical records of age, symptoms, and family history of breast cancer along with radiological and excision histology findings.
Imaging Evaluation and Biopsy
Mammographic images were obtained with dedicated film mammographic equipment (DMR, General Electric Medical Systems, Milwaukee, WI, USA) until April 2005 and two full-field digital mammography systems (Selenia, Lorad/Hologic, Danbury, CT, USA; Senogaphe DS, General Electric Medical Systems) after May 2005. Routine craniocaudal and mediolateral oblique views were obtained, and additional mammographic views were obtained as needed. US was performed by one of seven radiologists with 3-16 years of experience in breast imaging by using a high-resolution US unit with 5- to 10-MHz or - to 12-MHz linear-array transducers (HDI 5000 or 3000, Philips Advanced Technology Laboratories, Bothell, WA, USA; Logic 9, General Electric Medical Systems; or iU22, Philips Medical Systems, Bothell, WA, USA). All the breast US examinations were performed using handheld real-time US. Bilateral whole-breast scanning in every examination was performed for both screening and diagnostic purposes.
When microcalcifications were noted on mammography, a radiopaque marker was attached on the skin of the US noted lesion, the lesion which was to undergo subsequent biopsy. Additional routine craniocaudal and mediolateral oblique mammograms were conducted, and whether the microcalcifications on mammography were correlated with the US lesion or not was evaluated. The biopsy procedures were performed by one of seven radiologists with 3-16 years of experience in breast imaging. Tissue samples were obtained using a 14-gauge dual-action semiautomatic core biopsy needle (Stericut with coaxial, TSK Laboratory, Tochigi, Japan).
US-Guided VAE
The VAE procedure was performed using a vacuum-assisted device (Mammotome, Ethicon-Endosurgery, Cincinnati, OH, USA) with an 8- or an 11-gauge probe under the guidance of the high-resolution US unit mentioned above, as described by Kim et al. [10]. The VAE procedure was performed by one of four radiologists with 4-11 years of experience in breast imaging and US-guided biopsy.
Surgery and Follow-up
Surgery was performed by one of two breast surgeons with 9 and 16 years of experience in breast surgery, respectively. In cases of nonpalpable lesions, US-guided preoperative needle localization or US-guided preoperative skin marking was performed.
Data Collection and Statistical Analysis
After the review of the VAE and surgical results, the final diagnoses were categorized as either upgrade to malignancy (DCIS or invasive carcinoma) or benign (including high-risk lesions). An upgrade to malignancy of a pure MLL was determined when the finding of pure MLL was changed to malignancy at VAE or surgical pathology. The lesions that were not removed by VAE or surgery and that were stable after a year of follow-up after the biopsy were considered benign.
Images were reviewed in consensus by two radiologists with 3 and 16 years of experience in breast imaging. Mammographic features including mammographic breast density, mammographic findings, the presence of microcalcifications, and the morphology and distribution of microcalcifications on mammography were assessed. US features and the final assessment category according to the American College of Radiology’s Breast Imaging Reporting and Data System (BI-RADS) were assessed. The following US features were determined according to the terminology of the fifth American College of Radiology BI-RADS [11]: size, shape, margin, orientation, echo pattern, and posterior features. Regarding the echo pattern, a complex cystic and solid echoic mass was additionally classified as a complicated cyst, cyst with thin septations, cystic mass with thick wall and/or thick septations, intracystic mass (mixed cystic and solid appearance), and solid mass with eccentric cystic foci [12]. Complicated cysts could also have fluid-fluid or fluid-debris levels that might shift with changes as a result of patient position [11]. After reviewing both the mammographic and US features, the final assessment categorization was done on the basis of the combination of the two imaging studies. Category 4 was subclassified into 4A (low suspicion), 4B (moderate suspicion), and 4C (high suspicion).
Results
Among the 21 pure MLLs at CNB, the final pathology showed no upgrade to malignancy. All the 21 cases with pathology obtained by either VAE or surgery were benign MLLs including three cases of focal involvement of ADH (14.3%). The age range of the 21 patients with pure MLL at CNB was 27-76 years (mean, 45±11 years). There was no patient with a family history of breast cancer. Seventeen patients (81.0%) had no symptoms, and the lesions were detected on a screening examination; four patients (19.0%) were symptomatic. The symptoms were palpability (n=2), nipple discharge (n=1), and pain (n=1).
Mammography was available in 19 cases, and the findings are summarized in Table 1. The lesion size in the four cases with asymmetry or mass without microcalcifications on mammography, was 10-16 mm (mean, 12 mm), that in three cases with a diffuse distribution of microcalcifications was larger than 10 mm, and that in seven cases with grouped microcalcifications was 5-18 mm (mean, 9.2 mm). Microcalcifications were the most common mammographic feature (47.6%), and most of them were round in shape (50.0%) and had a grouped distribution (70.0%) (Fig. 1). The US features are presented in Table 2. The mean size of the lesions was 9.2±1.9 mm. The representative US features were oval shape (95.2%), circumscribed margin (76.2%), parallel orientation (90.5%), complex solid and cystic echo pattern (95.2%), and no posterior feature (76.2%). The most predominant BI-RADS category was category 4A (42.8%). Microcalcifications were shown on US in 10 cases (47.6%). In all cases, microcalcifications were located in the mass. The classification of complex solid and cystic echoic masses is presented in Table 3. Nine lesions (45.0%) showed intracystic mass (mixed cystic and solid appearance) (Fig. 1), and five lesions (25.0%) showed cystic mass with thick walls and/or thick septations. All the lesions showed image-pathologic concordance.

Mammographic features of pure mucocele-like lesions that underwent ultrasonography-guided 14-gauge core-needle biopsy
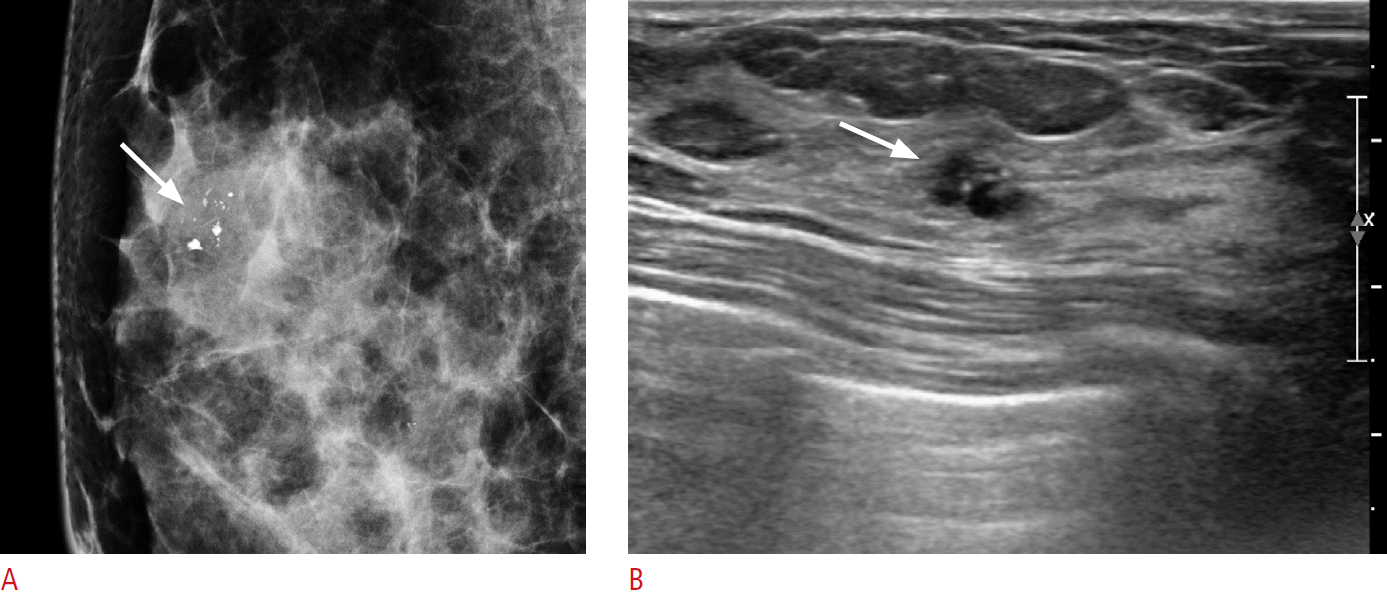
A 36-year-old woman with pure mucocele-like lesion (MLL).
A. A magnification mammography mediolateral view shows grouped coarse heterogeneous microlcalcifications in the right upper breast. B. A transverse sonogram shows a 7-mm oval-shaped circumscribed marginated complex cystic and solid echoic lesion showing an intracystic mass (mixed cystic and solid appearance) with microcalcifications. The lesion was categorized as Breast Imaging Reporting and Data System (BI-RADS) category 4A. The pathologic result of core biopsy was pure MLL with microcalcifications, and it was considered concordant with the imaging findings. The mass was surgically excised and was proven to be benign MLL on surgical pathology.
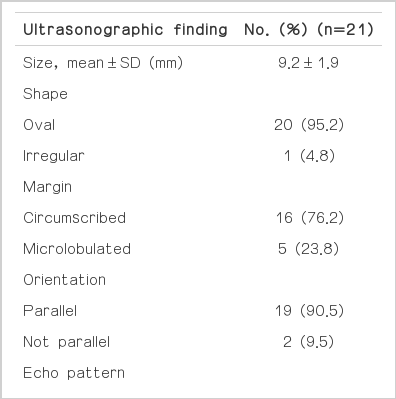
Ultrasonographic features for pure mucocele-like lesions that underwent ultrasonography-guided 14-gauge coreneedle biopsy
Discussion
Few publications have addressed pure MLL with in situ or invasive carcinoma as an upgrade to malignancy [1,9]. Our study defined and included MLLs that were upgraded into in situ or invasive carcinoma, since a diagnosis of ADH is not treated as MLL with in situ or invasive carcinoma. Further, including MLL with ADH as an upgrade could overestimate the risk associated with pure MLL diagnosed using US-guided 14-gauge CNB. The previous two studies on only pure MLL that was upgraded to DCIS show 0% and 4% upgrade rates, which is similar to our study [1,9].
Whether the microcalcifications can be a predictive factor to distinguish benign MLLs from malignant MLLs is controversial. Some suggest that the number, shape, and distribution of microcalcifications are not generally helpful in differentiating benign from malignant MLLs [2,13]. However, a 2005 study by Kim et al. [14] suggested that the microcalcifications of malignant MLLs extended over a wider area than those of benign MLLs. Our result correlated with that of Kim et al. [14]: a majority of microcalcifications of all the benign lesions in our study were clustered in the distribution (70.0%).
In terms of US features, it has been reported that US features do not help to differentiate between benign and malignant MLLs [15]. However, certain findings were predominant in the benign lesions considered in our study. The US feature of oval shape was the common finding in all the benign lesions in our study, and we think that this could be a distinguishing feature of benign and malignant MLLs (95.2%). This is supported by two previous studies suggesting the final pathology of mucinous carcinoma presenting as a well-defined or irregular-shaped solid mass on US and MLLs associated with DCIS presenting as irregular-shaped masses [2,7]. The circumscribed margin on US was the most common feature of the lesions considered in our study (76.2%). To the best of our knowledge, only one study, that by Carkaci et al. [2], has addressed the mass margin as the differential feature of benign and malignant MLLs. The study is in agreement with our study that the masses representing MLL with or without ADH had margins that were mostly circumscribed.
Although ultrasound elastography has not been performed in our study, it has been reported to be useful in differentiating benign and malignant breast masses, and this is supported by the fifth edition of the BI-RADS [11,16-18]. To the best of our knowledge, the efficacy of ultrasound elastography as a tool for distinguishing benign from malignant complex cystic and solid echoic masses has been reported by only one study, and a future study regarding its efficacy as a diagnostic tool is recommended [19].
As for the management of pure MLLs on US-guided CNB, controversy remains on whether CNB of an MLL is sufficient to diagnose benign pathology and thus, to avoid surgical excision [2]. The studies that pertain to surgical excision for the management of pure MLLs at CNB suggest a high upgrade rate of up to 43% [20- 22]. Such a high upgrade rate can be explained by the inclusion of lesions with ADH as an upgrade [4,5]. However, our study and the two previous studies that include only MLLs with in situ and invasive carcinoma as an upgrade, showed low upgrade rates of 0%-4.3%, indicating that surgical excision in all the cases of pure MLL at CNB is not desirable [1,9].
Previous studies have suggested that if vacuum-assisted biopsies have removed the entire radiological abnormality, in the setting of image-pathologic concordance, it may be possible to avoid surgical excision. Given the low upgrade rate and the fact that all the benign lesions in our study showed image-pathologic concordance, for lesions with image-pathologic concordance and the representative clinical and imaging features of benign MLLs, we agree with the suggestion of other authors that VAE should be conducted for complete removal of the radiologic abnormality and no further surgical excision should be carried out [3,5,6]. For only the lesions with image-pathologic discordance, we recommend surgical excision.
Our study had several limitations. First, the study was retrospective, and real-time US features were not available at the time of review; further, sonographic features were interpreted with static images. Although an expert in breast US performed all breast US, there might have been interobserver variability. To overcome this interobserver variability, two radiologists reviewed all of the static images retrospectively; however, some effect of the operator dependency of breast US could have remained. A prospective study is needed to overcome this limitation. Second, there was no case of upgrade to malignancy, and therefore, the findings of our study were not statistically supported. A further study with a larger population and a prospective design is needed.
In conclusion, depending on the features of a 0% upgrade rate to malignancy for all the 21 pathologically proven lesions in this study, a close imaging follow-up might be considered instead of surgical excision for pure MLL on US-guided CNB with image-pathologic concordance.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This study was supported in part by the Research Fund of the Korean Society of Ultrasound in Medicine.

