Ultrasonography of ovarian masses using a pattern recognition approach
Article information
Abstract
As a primary imaging modality, ultrasonography (US) can provide diagnostic information for evaluating ovarian masses. Using a pattern recognition approach through gray-scale transvaginal US, ovarian masses can be diagnosed with high specificity and sensitivity. Doppler US may allow ovarian masses to be diagnosed as benign or malignant with even greater confidence. In order to differentiate benign and malignant ovarian masses, it is necessary to categorize ovarian masses into unilocular cyst, unilocular solid cyst, multilocular cyst, multilocular solid cyst, and solid tumor, and then to detect typical US features that demonstrate malignancy based on pattern recognition approach.
Introduction
Ultrasonography (US) is the primary imaging modality for identifying and characterizing ovarian masses. US is a relatively simple and noninvasive diagnostic method that provides clinicians with useful information relevant for determining the optimal management strategy for a given patient. Lots of data have demonstrated that US can accurately characterize about 90% of adnexal masses and the reported sensitivity and specificity of US for detecting ovarian malignancies is 88%-96% and 90%-96%, respectively [1-3].
Various approaches have been used to characterize ovarian masses, including pattern recognition approach, simple scoring systems, statistically derived scoring systems, probability predictors based on logistic regression analysis, and complex mathematical models such as neural networks [4,5]. Of these, pattern recognition approach has been shown to have the advantage of combing easy interpretation with a higher accuracy than other methods for predicting malignancy [4,6,7].
However, the presence of significant variability in the terminology and definition of ultrasonographic findings in the above studies has led to the need for more standardization and uniformity in adnexal US. The International Ovarian Tumor Analysis (IOTA) framework is a pattern recognition approach that has been frequently used to present large-scale multicenter-based consensus results. It includes a standardized methodology for the US evaluation of ovarian masses and definitions of the ultrasonographic parameters of ovarian masses [6,8,9].
The various categories of ovarian masses according to the US features defined in the IOTA framework are reviewed in this article.
Definitions of Ultrasonographic Features
A septum is defined as a thin strand of tissue running across the cyst cavity from one internal surface to the contralateral side [9]. An incomplete septum is defined as a thin strand of tissue running across the cyst cavity from one internal surface to the contralateral side without being complete in some scanning planes [9]. If a cyst has more than one complete septum, it is classified as multilocular. A cyst with no complete septum, is classified as unilocular.
Solid refers to an object that exhibits a higher echogenicity than that of the adjacent myometrium, and the blood flow of solid tissue can be detected on Doppler US. Diffuse wall thickening of a cyst, ovarian stroma, blood clots, fat, and regular septa are not regarded as solid tissue. In particular, solid papillary projections are defined as any solid projections into the cyst cavity from the cyst wall with a length greater than or equal to 3 mm [9]. If there is at least one solid papillary projections, the wall is defined as irregular. Otherwise, the wall is defined as smooth. All ovarian masses can be placed in one of five categories according to the presence of septa and a solid component as follows:
- Unilocular cyst: a cyst without septa and a solid component
- Unilocular solid cyst: a unilocular cyst with a solid component
- Multilocular cyst: a cyst with at least one septum but no solid component
- Multilocular solid cyst: a multilocular cyst with a solid component
- Solid tumor: a tumor where the solid components comprise 80% or more of the tumor when assessed in two-dimensional section
Unilocular Cyst
Most follicular cyst, hemorrhagic corpus luteal cyst, benign serous tumor, endometrioma, and cystic teratoma are categorized as unilocular cyst. There is no specific imaging feature for the diagnosis of tumorous unilocular cyst. Age, size, and morphological changes of the mass in a follow-up examination can be important factors in distinguishing between tumorous and nontumorous unilocular cyst [10,11].
Most unilocular cyst such as follicular cyst and benign serous tumor can demonstrate anechoic features (Fig. 1). In large unilocular cyst, it is important not to overlook small papillary projections, although unilocular cyst has a very low risk of malignancy of 0.96% [12]. Since blood clots, fat, and sebaceous materials can be occasionally mistaken for solid components within a cyst, it is necessary to understand the typical US features of “complex” cyst, which is defined as cyst containing any kind of nonviable components. Examples of complex cyst are hemorrhagic corpus luteal cyst, endometrioma, or mature cystic teratoma.
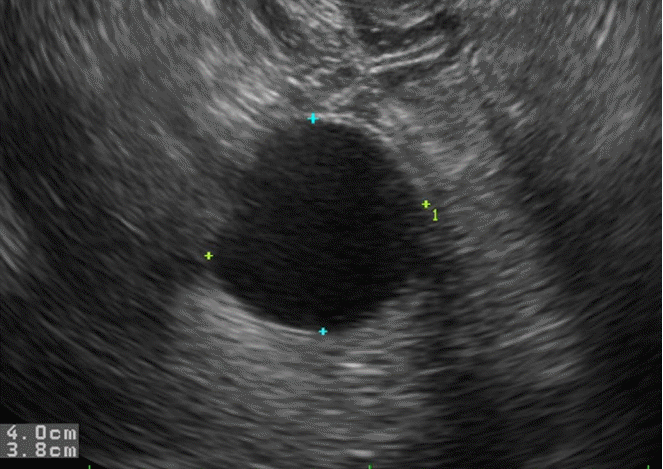
Follicular cyst in a 22-year-old woman.
Transvaginal ultrasonography shows a well defined anechoic mass without a solid component.
Hemorrhagic corpus luteal cyst can be caused by bleeding to corpus luteum. In the acute stage, a hemorrhagic cyst may contain clotted blood, which manifests in US as intensely echogenic, avascular, homogeneous or heterogeneous material. However, clotted blood does not show vascularity. It can occasionally appear to have a bizarre contour compared to the lobulated contour found in malignancies (Fig. 2) [4,13]. Over time, the clot may retract and liquefy, resulting in an undulating and concave surface. In a later stage, resolved clots with fibrin strands result in a pattern that referred to with a variety of terms, including “cobweb,” “honeycomb,” “reticular,” “lacy,” “fishnet,” and “sponge” (Fig. 3) [4]. Fibrin strands are usually seen as avascular irregular thin lines that do not completely transverse the cyst, whereas septa are seen as thicker linear echoes with vascularity [14-16].
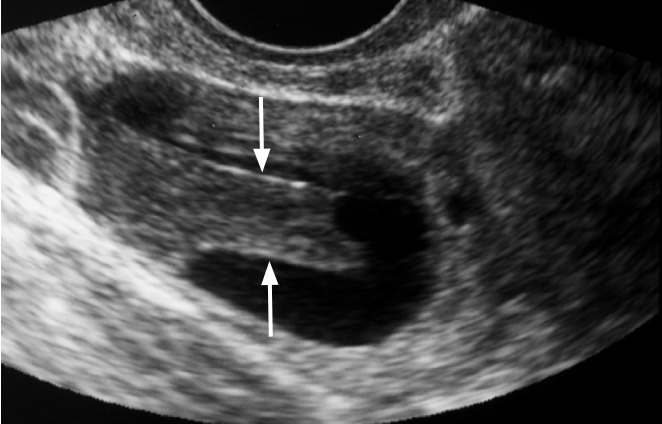
Hemorrhagic corpus luteal cyst in a 25-year-old woman.
Transvaginal ultrasonography (US) shows a rectangular hypoechoic lesion in the cystic mass (arrows). The lesion had no demonstrable flow on Doppler US (not shown).

Hemorrhagic corpus luteal cyst in a 23-year-old woman.
Transvaginal ultrasonography shows a round complex echoic ovarian mass. A reticular pattern in the internal echo due to fibrin strands is visible (arrows).
Endometrioma occurs in the ovary where ectopic endometrial tissue is implanted. The characteristic US features of endometrioma are homogeneously diffuse low-level echoes in the cyst, compromising the so called ground-glass appearance, which is indicative of chronic repetitive hemorrhages within the cyst (Fig. 4) [17]. However, less than 15% of endometrioma have atypical findings, such as fluid-fluid level, hyperechoic mural irregularity, heterogeneity, or calcification (Fig. 5) [18,19]. In addition, endometrioma in postmenopausal patients are less likely to exhibit the typical “ground-glass” pattern of echogenicity, and malignant transformations to endometrioid or clear cell carcinoma have been reported in older patients (Fig. 6). Therefore, endometrioma in elderly patients should be carefully evaluated in order to exclude malignancy.
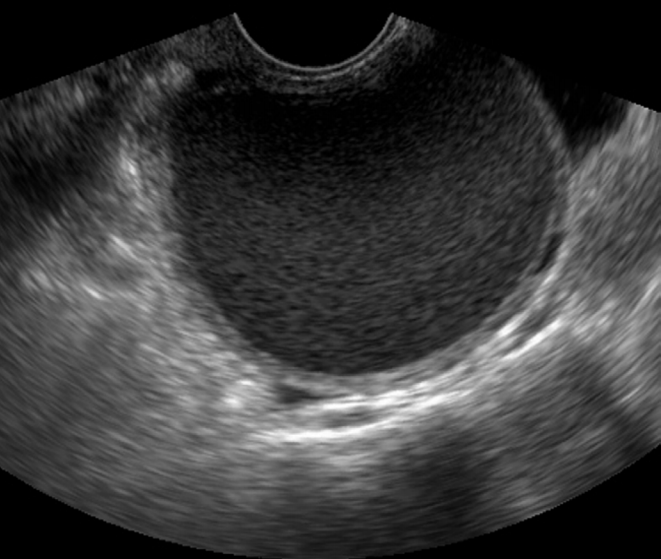
Typical endometrioma in a 32-year-old woman.
Transvaginal ultrasonography reveals homogeneously diffuse low echoes in the cystic mass, which is known as “ground-glass” appearance.
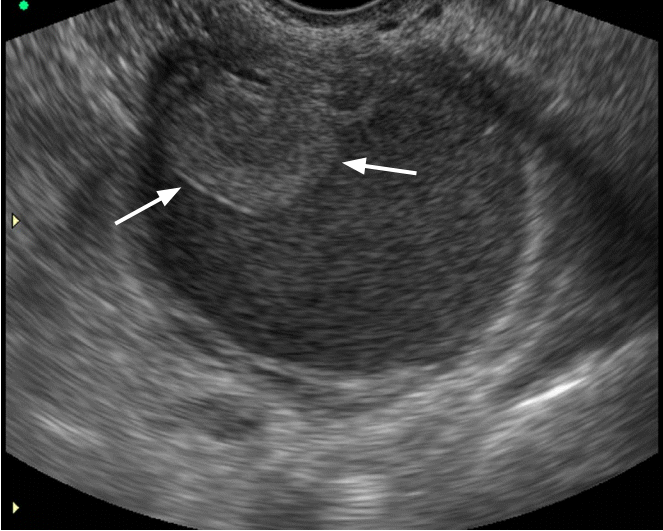
Atypical endometrioma in a 29-year-old woman.
Transvaginal ultrasonography (US) reveals a well defined round lesion within a homogeneously hypoechoic cyst (arrows). Doppler US demonstrated no blood flow in the lesion, which proved to be a localized blood clot after surgical resection (not shown).

Endometrioid carcinoma on background of endometriosis in a 44-year-old woman.
Transvaginal ultrasonography shows several small polypoid lesions that have diffuse internal low echoes (arrows) along the wall of the cystic mass.
Mature cystic teratoma, often referred to as dermoid or dermoid cyst, typically shows focal high echogenic nodules, heterogeneous internal echoes in the cyst with acoustic shadows, and multiple hyperechoic fine lines and dots, which are due to reflection by clumps of hair, sebum, or fat component within the mass (Fig. 7) [20-22]. The hyperechoic area is not usually as intensely echogenic as calcification and may be confused with the echo of adjacent bowel gas (Fig. 8) [4]. In some cases, atypical features such as fluidfluid level, anechoic cyst, and multiple floating globules can be detected [23-25].
Mature cystic teratoma in a 37-year-old woman.
Transvaginal ultrasonography demonstrates a complex echoic mass with a hyperechoic solid portion and posterior shadowing (arrows). The mass also contains multiple hyperechoic lines and dots.
Unilocular Solid Cyst
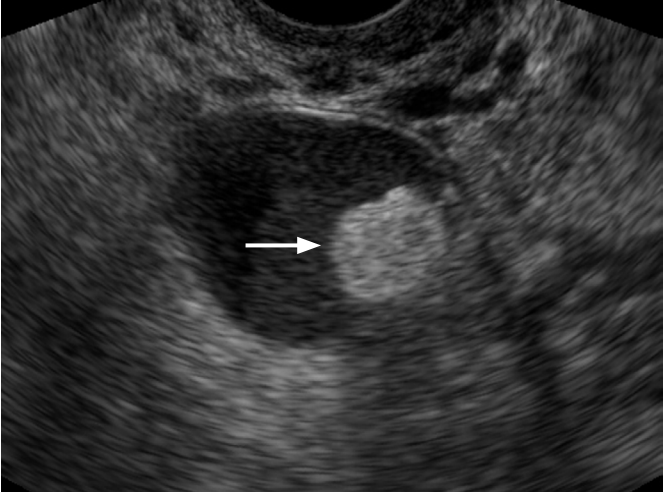
Most serous cystadenocarcinoma, endometrioid adenocarcinoma, clear cell carcinoma, serous borderline malignancies, and cystadenofibroma are categorized as unilocular solid cyst. According to a study using IOTA framework, the identification of a unilocular solid cyst has a positive predictive value for malignancy of 37.1%, a sensitivity of 16.1% and a specificity of 90.0% [26]. This type of cyst contains one of the typical features of ovarian malignancies. Single or multiple papillary projections of the cystic wall can exist as the solid component of serous cystadenocarcinoma or endometrioid adenocarcinoma, where vascular flow can be detected (Fig. 9).
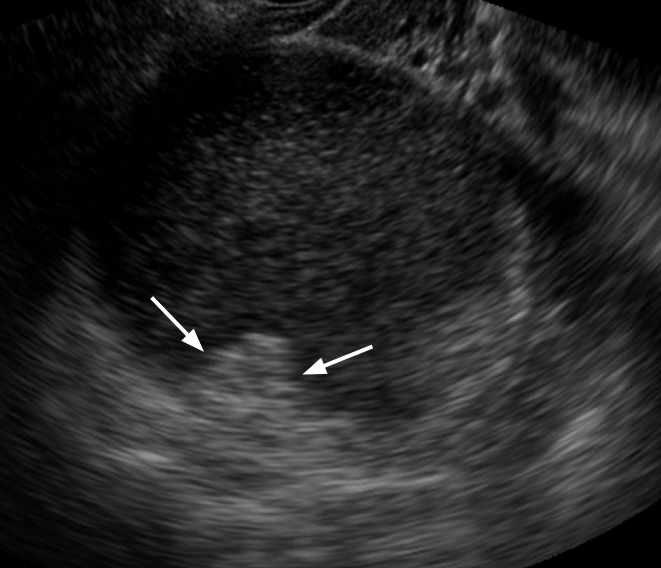
Serous cystadenocarcinoma in a 59-year-old woman.
A unilocular cystic mass with internal papillary projection is seen on transvaginal ultrasonography (arrows).
Clear cell carcinoma usually appears as a large cystic mass with a smooth marginated solid component (Fig. 10) [27]. Cystadenofibroma is a benign and rare type of ovarian tumor that has a mixture of epithelial and fibrous stromal components and can contain have papillary projections or solid nodules within a cyst [28].

Clear cell carcinoma in a 60-year-old woman.
Transvaginal ultrasonography (US) shows a well defined cystic mass with a solid nodule which has a smooth round margin and seems to be slightly different from typical papillary projections (arrows). Doppler US demonstrated blood flow in the solid nodule (not shown).
Multilocular Cyst
Most mucinous cystadenoma, endometrioma, theca lutein cyst, mature cystic teratoma, tubo-ovarian abscess, and most cases of polycystic ovarian syndrome, ovarian hyperstimulation syndrome, and mucinous borderline malignancies are categorized as multilocular cyst.
Mucinous cystadenoma usually appears as multilocular cysts containing different echogenic materials, ranging from anechoic to diffuse low echogenic with floating echogenic foci that suggest a variety of mucin components (Fig. 11) [10,11].

Mucinous cystadenoma in a 47-year-old woman.
Transvaginal ultrasonography shows a thin walled multilocular cystic mass with a regular septum.
Theca lutein cyst reflects a form of ovarian hyperstimulation resulting from abnormally elevated levels of serum beta-human chorionic gonadotropin, accompanying multiple gestations, hormonal stimulation for assisted reproductive techniques, or molar pregnancies [29,30]. They are mostly frequently bilateral.
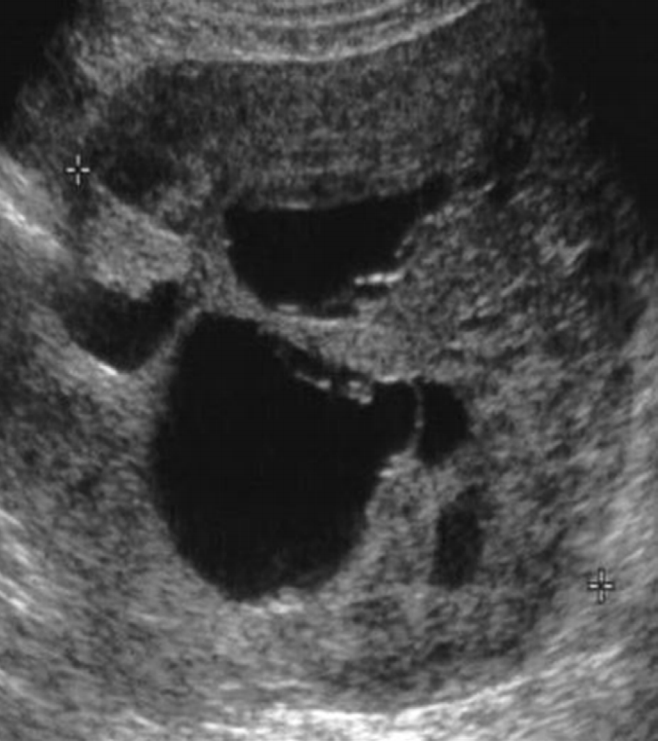
Tubo-ovarian abscess is manifested as complex multilocular cyst with thick walls and thick septa, filled with homogeneously diffuse low echoic materials [31,32]. As the disease progresses, the walls and septa can be changed to be thicker with more increased vascularity. Therefore, one should keep in mind that the above type of cyst may be confused with ovarian malignancies (Fig. 12).
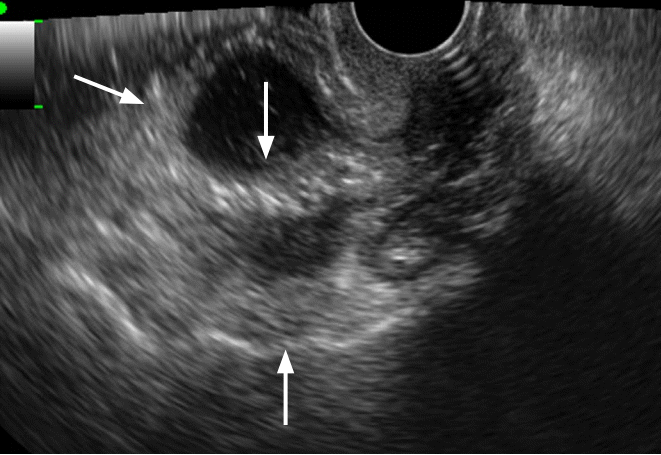
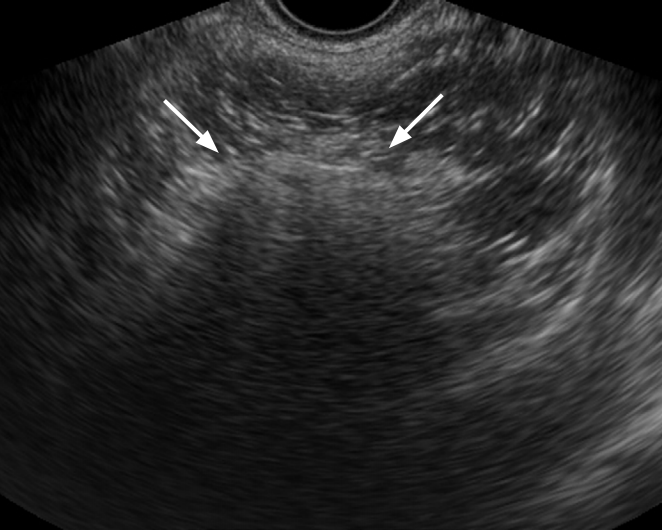
Tubo-ovarian abscess in a 35-year-old woman.
Transvaginal ultrasonography shows a thick walled multilocular cystic mass with internal echogenic debris. Regular thickening of the outer wall and the septum of the cystic mass should be noted (arrows).
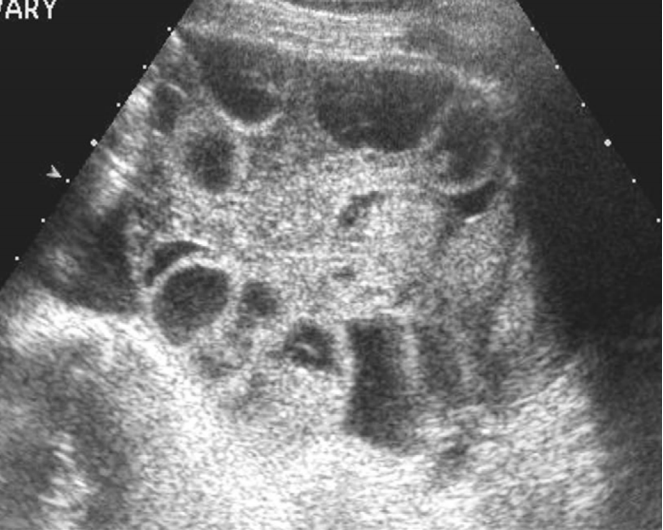
Polycystic ovarian syndrome or disease is a type of anovulatory hormonal disorder. Its features include the presence in one or both ovaries of 12 or more follicles measuring 2 mm to 9 mm, or an ovarian volume exceeding 10 mL [33,34]. Such features are caused by ovarian stromal hyperplasia and the decrease of follicular stimulating hormone. The presence of polycystic ovaries alone in the absence of clinical signs such as amenorrhea, infertility, obesity, and hirsutism is insufficient for diagnosing this syndrome.
Ovarian hyperstimulation syndrome is an iatrogenic complication following ovulation stimulation in patients treated for infertility. Both ovaries may demonstrate cystic enlargement with ascites or pleural effusion (Fig. 13) [35,36].
Multilocular Solid Cyst
Most mucinous cystadenocarcinoma, endometrioid adenocarcinoma, mucinous borderline malignancies, cystadenofibroma, struma ovarii, and granulosa cell tumor are categorized as multilocular solid cyst. According to a study using the IOTA framework, the identification of a multilocular solid cyst has a positive predictive value for malignancy of 43.0%, a sensitivity of 42.1%, and a specificity of 79.6% [26]. This type reflects one of the typical features of ovarian malignancies.
Mucinous cystadenocarcinoma is commonly unilateral and involve the presence of irregular thick septa or solid papillary projections that are usually detected within cysts (Fig. 14).

Mucinous cystadenocarcinoma in a 65-year-old woman.
Transvaginal ultrasonography shows a multilocular cystic mass with large papillary projections (arrow).
Struma ovarii is a rare type of tumor that is considered to be a type of monodermal teratoma of the ovary. The most characteristic US feature of this entity is the presence of well-defined solid tissue with a smooth margin that is vascularized on Doppler US (Fig. 15) [37-39]. Its solid components are likely to correspond to the microfollicular or fibrous area of the tumor [40].
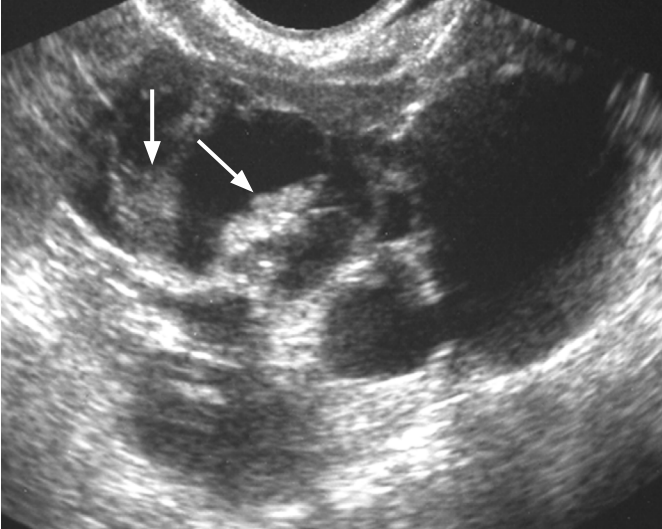
Struma ovarii in a 37-year-old woman.
Transvaginal ultrasonography shows a multilocular cystic mass and internal solid components with various echoes (arrows).
Granulosa cell tumor is a type of sex cord-stromal tumors. This tumor has two typical patterns: one typical pattern is a multilocular solid cyst containing a considerable amount of solid tissue surrounding relatively small locules without papillary projections, resulting in a so-called Swiss cheese or honeycombing appearance (Fig. 16). Histopathologically, this type of tumor has a predominantly macrofollicular pattern with multiple cystic spaces and hemorrhagic areas interspersed by thickened septa. The second typical pattern involves a heterogeneously echoic solid mass with necrosis. Histopathologically, this appearance is ascribed to evenly distributed trabecular or diffuse patterns in the tumor cells without cystic changes [40,41].
Solid Tumor
Most sex cord-stromal tumor such as fibroma, fibrothecoma, granulosa cell tumor, sclerosing stromal tumor, surface epithelial tumor such as Brenner tumor, serous papillary carcinoma, malignant germ cell tumor such as dysgerminoma, endodermal sinus tumor, lymphoma, and metastatic tumor are categorized as solid tumor. According to a study employing IOTA framework, the identification of a solid tumor has a positive predictive value for malignancy of 65.3%, a sensitivity of 33.6%, and a specificity of 6.6%. When evaluated with a pattern recognition technique, this type of tumor has a positive likelihood ratio for malignancy of 5.09, which is the highest of any of the above-described categories of ovarian masses [26].
However, some benign tumor does appear as solid masses. Fibroma and fibrothecoma are typical cases. Those are ovarian tumors arising from the spindle cells or stromal cells that resemble theca cells [42]. The characteristic US features of fibroma and fibrothecoma include the presence of a round, oval, or lobulated hypoechoic mass with minimal to moderate vascularity (Fig. 17), whereas cystic or hemorrhagic change is only detected in rare cases [43]. Brenner tumor is a rare type of ovarian epithelial tumor composed of epithelial nests that are surrounded by proliferating dense stromal tissue [44]. Brenner tumor usually has US feature similar to those of fibroma and fibrothecoma. They occasionally have multiple calcifications (Fig. 18) [44,45]. Sclerosing stromal tumor is a type of rare benign tumor that usually occurs in young patients. The major US feature of sclerosing stromal tumor is the presence of a solid or multilocular solid mass with marked peripheral vascularity [46,47].
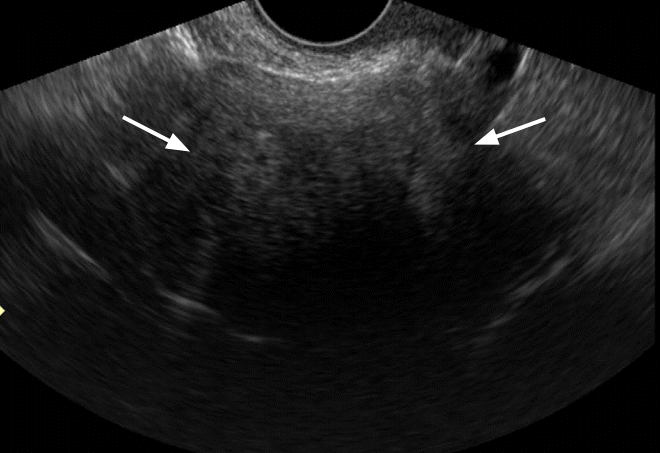
Fibroma in a 50-year-old woman.
Transvaginal ultrasonography shows a homogeneously hypoechoic solid mass (arrows) with strong posterior shadowing.
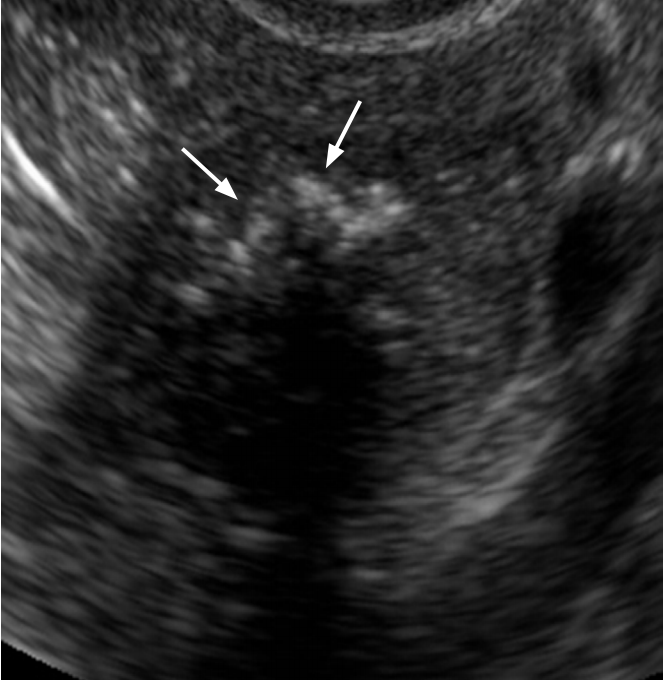
Brenner tumor in a 49-year-old woman.
Transvaginal ultrasonography shows a hypoechoic solid mass with punctate echogenic foci (arrows).
Serous papillary carcinoma is usually detected as an irregular solid mass spreading along the surface of the ovary with a supporting structure or peritoneum. Interestingly, due to this pattern of this tumor’s growth and spread, the ovarian stroma may appear relatively normal despite of its extensive involvement in peritoneal carcinomatosis [10,48].
Malignant germ cell tumors include dysgerminoma and endodermal sinus tumor. These are large solid tumors that are more common in younger women. In particular, dysgerminoma appear as large lobulated solid tumor with prominent fibrovascular core and minimal necrosis (Fig. 19) [49].
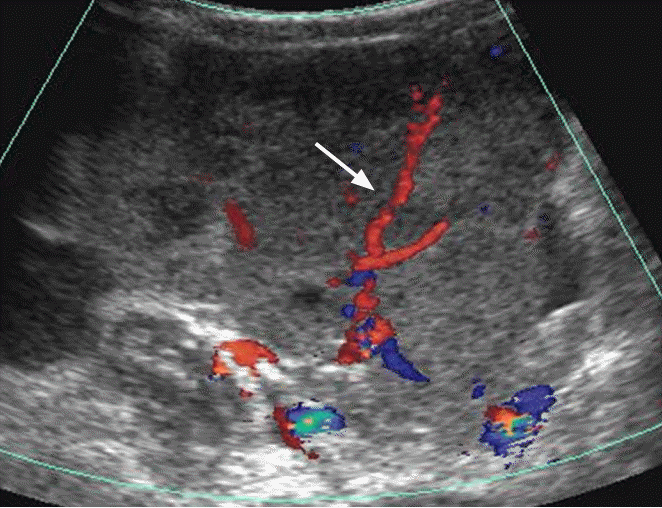
Dysgerminoma in a 41-year-old woman.
Transabdominal Doppler ultrasonography shows a lobulated hypoechoic solid mass with prominent blood flow in the fibrovascular septa (arrow).
When bilateral predominantly solid ovarian masses are seen, metastatic ovarian tumors or lymphoma should be considered. Primary ovarian lymphoma is a rare manifestation of non-Hodgkin’s lymphoma (NHL) accounting for 0.5% of NHL and 1.5% of ovarian tumors. The tumor is commonly seen as homogeneous solid masses with increased vascularity (Fig. 20) [50]. Metastasis to the ovary accounts for 5%-20% of ovarian tumors. Common primary sites of metastatic ovarian tumors include the gastrointestinal tract, breast, and uterus [51,52]. Krukenberg tumor is traditionally defined as ovarian metastatic tumor composed of mucin-filled signet ring cells associated with the proliferation of cellular non-neoplastic stroma originating from the stomach, colon, cecum, appendix, or breast (Fig. 21). However, some researchers have classified any kind of ovarian tumor metastasizing from any site Krukenberg tumor. Metastases derived from the stomach or breast appear as well defined solid masses with some necrosis. In contrast, metastases derived from the colon, rectum, appendix, or biliary tract are most often multilocular or multilocular solid [53].
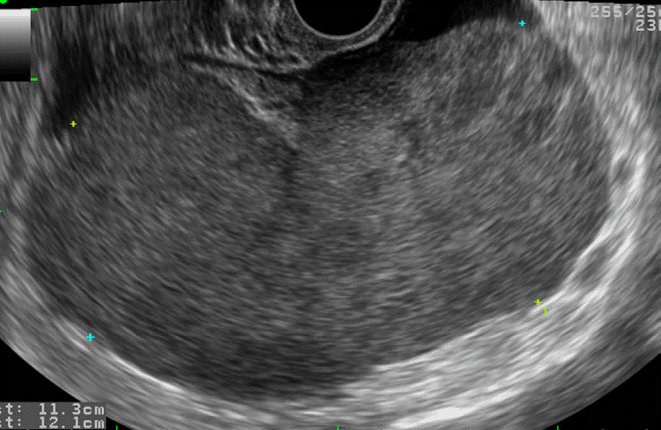
Ovarian lymphoma in a 59-year-old woman.
Transvaginal ultrasonography shows a hypoechoic solid mass without necrosis or hemorrhage.
Conclusion
The accurate characterization of ovarian masses is a common challenge in clinical practice. In order to create an easy and simple classification facilitating the differentiation of benign and malignant masses, familiarity with the pattern recognition approach to US is required. The pattern recognition approach described in this study can be acquired without particular difficulty by anyone who performs gynecological US examinations on a regular basis. Using the categories of unilocular cyst, unilocular solid cyst, multilocular cyst, multilocular solid cyst, and solid tumor, it is possible to recognize typical cases of each category. This results in confident and specific diagnoses of hemorrhagic corpus luteal cyst, cystic teratoma, endometrioma, tubo-ovarian abscess, benign or malignant epithelial tumor, sex cord stromal tumor, rare malignant germ cell tumor, and metastatic tumor.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This study was supported by the 2011 Philips Scholarship Fund through the Korean Society of Ultrasound in Medicine.