Photoacoustic imaging platforms for multimodal imaging
Article information
Abstract
Photoacoustic (PA) imaging is a hybrid biomedical imaging method that exploits both acoustical and optical properties and can provide both functional and structural information. Therefore, PA imaging can complement other imaging methods, such as ultrasound imaging, fluorescence imaging, optical coherence tomography, and multi-photon microscopy. This article reviews techniques that integrate PA with the above imaging methods and describes their applications.
Introduction
Photoacoustic (PA) imaging is a hybrid biomedical imaging method that exploits both acoustical and optical properties. PA imaging has been evaluated as a preclinical and clinical imaging method in the biomedical field [1-6]. PA imaging is based on the PA effect [7]. When a pulsed laser with a pulse width of a few nanoseconds illuminates a target object, a PA wave can be induced as a consequence of the object’s thermoelastic expansion and subsequent relaxation (Fig. 1) [2]. An ultrasound (US) transducer detects the PA wave, and an image is reconstructed through an imaging process. The initial pressure P (Pa) of a generated PA wave can be expressed as
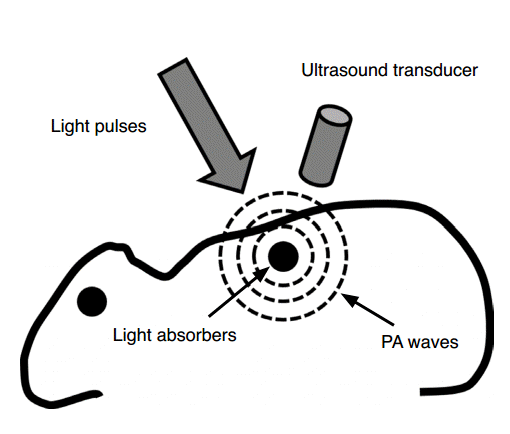
Schematic of photoacoustic imaging.
When a target absorbs pulsed light, photoacoustic (PA) waves are generated via thermoelastic expansion. The PA waves propagate and are detected by an ultrasound transducer. Reprinted from Kim et al. Chem Rev 2010;110:2756-2782 [2], with permission of American Chemical Society.
where Γ is the temperature-dependent Grueneisen parameter, σ (0≤σ≤1) (dimensionless) is the heat conversion efficiency, μa (1/cm) is the optical absorption coefficient, and F (W/m2) is the optical fluence. All of these parameters are directly proportional to PA amplitude, and therefore increasing one or more of the parameters can increase PA amplitude.
The PA wave equation can be expressed as
where vs (m/sec) is the sound velocity in the medium, p(
PA imaging integrates the advantages of US and optical imaging. The penetration depth of PA imaging is up to 8 cm, whereas that of pure optical imaging techniques is up to 1 mm. PA imaging does not have speckle artifacts, whereas pure US does. PA imaging can also provide physiological information such as hemoglobin concentration, hemoglobin oxygen saturation, and angiogenesis. Therefore, PA imaging can provide additional complementary information when combined with other imaging methods such as US [22], fluorescence (FL) [23], optical coherence tomography (OCT) [24], and multiphoton microscopy (MPM) [25]. This review will describe techniques that integrate PA with the above imaging methods and discuss their applications.
US and PA imaging
US imaging is widely used in the biomedical field because it uses nonionizing acoustic radiation and can therefore provide real-time in vivo images without any risk of damage. However, its images suffer from intrinsic speckle artifacts that degrade the image quality. PA images do not contain speckle artifacts, so high-contrast images can be acquired. PA imaging can provide physiological information, such as hemoglobin concentration, hemoglobin oxygen saturation, and angiogenesis, as well as structural information. Therefore, PA imaging can provide information complementary to US when the two techniques are used in combination.
Due to its intrinsic properties, PA imaging can be easily integrated with clinical US machines [10-13,26-29]. In order to acquire a PA image, the radio frequency signal sequences of a clinical US machine must be modified. In US imaging, a transducer transmits US waves and receives the reflected US echoes. However, in PA imaging, the laser irradiation induces acoustic waves in the medium, so the US transducer only needs to receive the generated PA waves, instead of transmitting US waves. Therefore, the transmitting function of a clinical US system must be blocked in order to acquire a PA image. Furthermore, the beamforming algorithm must be modified to enable one-way detection. In order to modify the beamforming algorithm, the raw data of the received PA signal must be accessible. Thus, a US system can be modified to provide PA/US imaging ability by simply adding a pulsed laser source (Fig. 2A) [10].
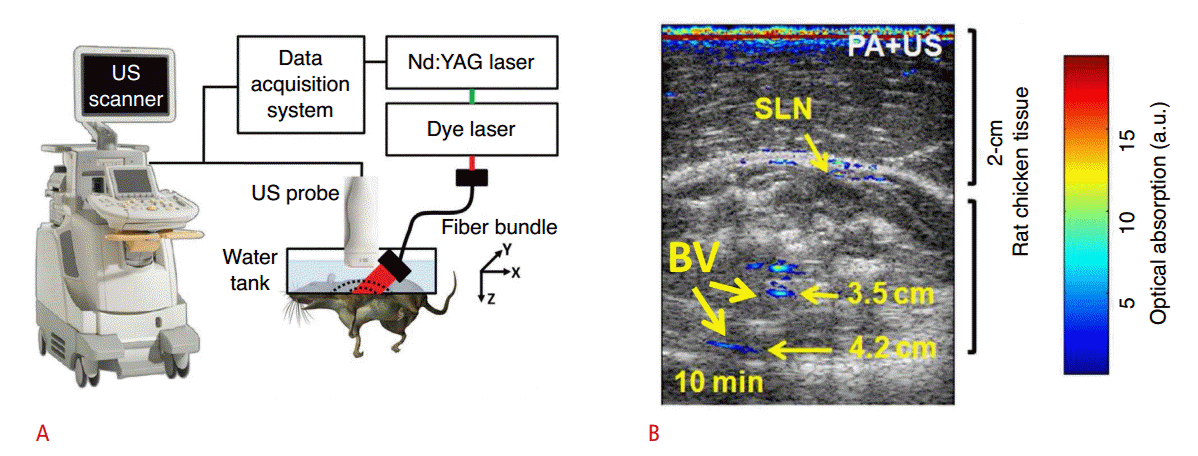
Experimental setup of PA/US imaging system and example image.
A. Schematic of integrated PA/US imaging system. The wavelength of the pumped laser is tuned by a dye laser and coupled with a fiber bundle. The fiber bundle is integrated with a US probe and excites target tissues. The generated PA waves are detected by US probe and saved by data acquisition system. B. The PA/US image of the SLN of the rat in vivo. Grayscale represents the sonogram and pseudo-color represents the PA image. PA signals from the SLN and BVs are indicated in (B). Nd:YAG, neodymium-doped yttrium aluminum garnet; PA, photoacoustic; US, ultrasound; SLN, sentinel lymph node; BV, blood vessel. Reprinted from Erpelding et al. Radiology 2010;256:102-110 [10], with permission of Radiological Society of North America; reprinted from Kim et al. Biomed Opt Express 2010;1:278-284 [11], with permission of The Optical Society.
The wavelength of a neodymium-doped yttrium aluminum garnet pump laser is tuned by a dye laser, so that the user can select an appropriate wavelength of light for obtaining PA signals of the target object. The output of the laser is coupled with a bifurcated fiber bundle, which is adapted with a US transducer probe. Pulsed laser beams are delivered by the fiber bundle to excite the target object. The excited object generates PA waves, which propagate omnidirectionally, such that the US transducer can receive them. The received PA signals are acquired by a data acquisition system and processed to construct a PA/US image. The integrated PA/US imaging system can provide both functional and structural information about biological tissues.
The sentinel lymph node of a rat was imaged in vivo using an integrated dual-modal PA/US imaging system (Fig. 2B) [11]. Methylene blue was injected into the forepaw to increase optical absorption contrast in the sentinel lymph node, and PA and US images were then acquired and overlaid. In order to increase the imaging depth, 2 cm of chicken tissue was laid on the rat. Pseudocolor was used to represent the PA image that shows the optical absorption contrast, and grayscale was used to represent the US image that shows the structure of surrounding tissues. The sentinel lymph node and major blood vessels were clearly distinguishable in the PA image, and the internal structures of the rat were visible in the US image. The combined PA/US image provided both functional information, such as methylene blue accumulation in the sentinel lymph node, and structural information, such as the shape of the surrounding tissue.
By exploiting well-developed US technology, PA imaging capability can be easily added to a clinical US imaging scanner. Typically, US imaging provides morphological information whereas PA imaging supplies physiological information.
FL and PA imaging
FL imaging is a nonionizing and inexpensive technique with applications in molecular imaging [23,30-32]. However, due to optical diffusion in biological tissues, the spatial resolution of FL imaging is poor when light penetrates beyond 1 mm. In contrast, PA imaging retains spatial resolution to a penetration depth >5 cm. In general, the combination of FL and PA imaging uses exogenous contrast agents to visualize complementary optical absorption and FL properties of target objects. When irradiated by a laser source, an object gains energy, and then releases it either as light of another wavelength (i.e., FL) or as heat (i.e., a PA wave). The energy of FL depends on the FL quantum yield Y; the PA wave is generated by the remaining energy (i.e., 1-Y) [2]. Therefore, both PA and FL images of a target object can be acquired by injecting a single contrast agent that has an appropriate Y, so that the signal can be detected by both the FL and the PA imaging systems.
Indocyanine green has been used in combined PA and FL imaging [33,34]. In vivo PA and FL images of the bladder in rats were acquired after injecting indocyanine green [33]. The images were taken by separate PA and FL imaging systems (Fig. 3A, B). In the PA imaging system, pulsed laser beams with a pulse duration of 5 nanoseconds were generated by a 10-Hz Q-switched neodymiumdoped yttrium aluminum garnet pump laser. The generated laser beams were tuned using an optical parametric oscillator system. The wavelength of the output laser can be tuned from 680 to 2,500 nm. The wavelength-tuned laser was delivered to a conical lens to make a donut-shaped beam pattern. A single-element focused US transducer was then placed in the center of the ring-shape beam. The diverged beam was refocused on the target object with an optical condenser. A bottom-open water tank and US gel were used to match acoustic impedance between the rat and the US transducer. The bottom of the water tank was sealed using a transparent membrane. The PA waves were detected by a US transducer, amplified, and saved by a data acquisition system. Mechanical raster scanning was used to acquire volumetric data. The PA image was constructed by postprocessing the saved data. In the FL imaging system, a continuouswave laser source was used. The output laser was filtered by an excitation filter, and then beamed directly at the target. The emitted FL signals were filtered by an emission filter and focused on a charge-coupled device camera, which detected the FL signals and constructed the FL image. Using this combination of PA and FL, in vivo bladder FL and PA images of the rat were acquired (Fig. 3C, D). Indocyanine green was injected through a 22-gauge lubricantcoated catheter. In general, the bladder itself does not have optical absorption, and is therefore invisible in a PA image. After injecting indocyanine green, the resulting PA image clearly showed the shape of bladder, as well as surrounding blood vessels (Fig. 3C). The FL image did not provide structural information about the bladder but could verify that it took up indocyanine green (Fig. 3D).
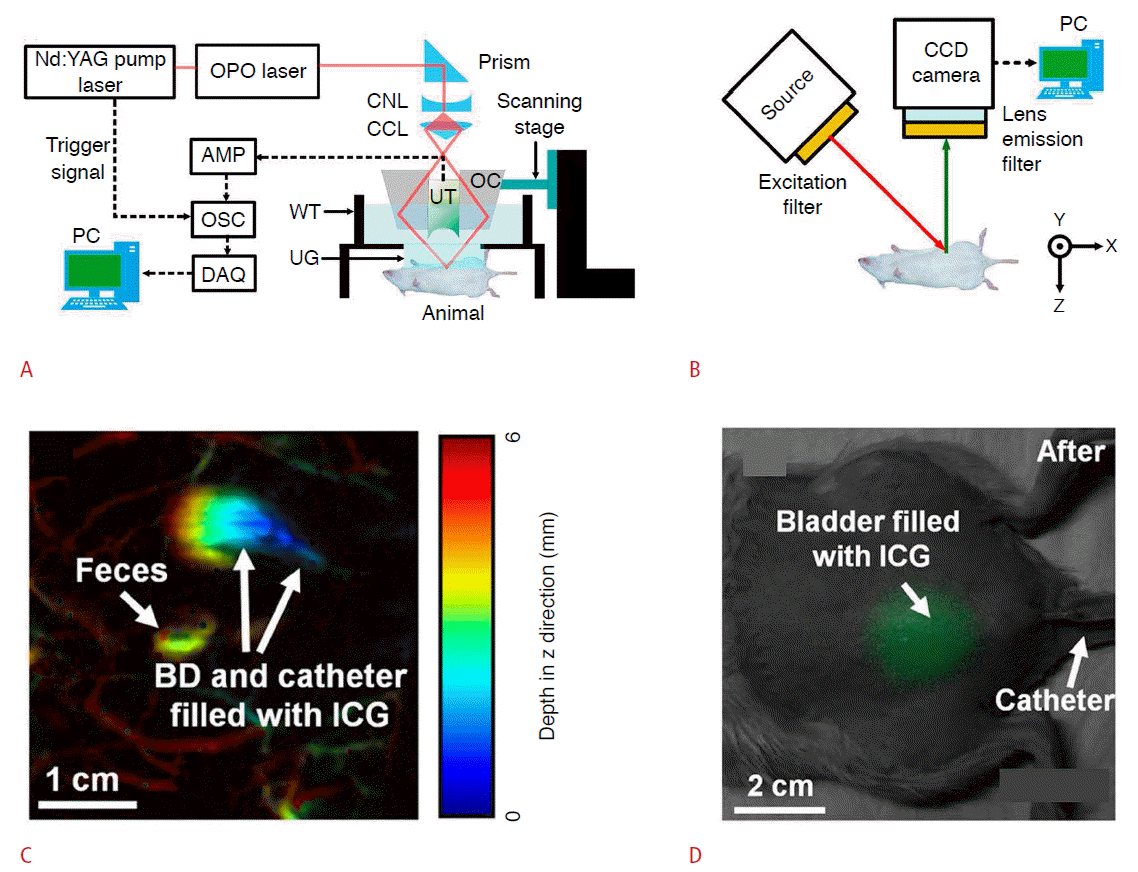
Experimental setup of PA and FL imaging systems, and example image of each modality.
A. The wavelength of pumped laser is tuned by OPO laser and guided to CNL. CNL and CCL produce a ring-shaped beam and the OC merges the diverged beam to the target object. The UT detects the generated PA waves and the DAQ saves the detected signal. B. A filtered source laser excites the target tissue and the CCD camera detects the emitted FL signal. The FL signal is filtered by an emission filter. C. In vivo noninvasive PA images of an ICG injected bladder in a rat. Pseudo-color represents depth information (red=deep to blue=shallow). The PA signal from ICG in the bladder and catheter is clearly visible in (C). D. In vivo noninvasive PA images of ICG injected in the bladder of a rat. The grayscale picture is acquired under room light, and the green color represents the FL signal of the ICG. The background picture and FL image are acquired separately and overlaid in post-processing. Nd:YAG, neodymium-doped yttrium aluminum garnet; OPO, optical parametric oscillator; CNL, conical lens; CCL, concave lens; AMP, amplifier; OSC, oscilloscope; DAQ, data acquisition system; OC, optical condenser; UT, ultrasound transducer; WT, water tank; UG, ultrasound gel; PC, personal computer; BD, bladder; ICG, indocyanine green; PA, photoacoustic; FL, fluorescence. Reprinted from Park et al. Sensors (Basel) 2014;14:19660-19668 [33], according to the Creative Commons License MDPI.
Due to its low cost and use of nonionizing radiation, FL imaging has been evaluated for use in molecular imaging. However, due to strong light scattering, FL suffers from either poor spatial resolution or shallow penetration. Combined PA and FL imaging may become a vital technique in molecular imaging because it is safe, sensitive, inexpensive, and has high resolution.
PAM and OCT
PAM and OCT are both noninvasive microscopic optical imaging methods that exploit optical absorbance and optical scattering, respectively [35,36]. OCT uses a Michelson interferometer to perform low-coherence interferometry and can provide microstructural information with a resolution of 1-10 μm with an imaging depth of a few millimeters. OCT can also measure blood flow by exploiting the Doppler effect and optical polarization. Complementary information obtained using optical absorption and optical scattering can be provided by combining PAM and OCT imaging. In the integrated image, PAM provides functional information such as hemoglobin oxygen saturation, and OCT provides structural information about surrounding microstructures [35,37-39]. The most practical applications of the integrated PAM/OCT imaging system are in otolaryngology and ophthalmology. Conventional OCT has been predominantly used in ophthalmology because the optical transparency of the eye’s cornea and lens greatly enhances the OCT imaging depth. In ophthalmology, OCT exploits optical scattering to provide structural information about the cornea, iris, and retina [40]. Further, OCT can even detect the multilayer structure in the retina, which is useful for diagnosing diabetic retinopathy. An integrated PAM/OCT imaging system can also provide optical absorption contrast information, such as retinal blood vessel distribution and hemoglobin oxygenation; this information can be useful in the diagnosis of retinal telangiectasia and Coats disease [41,42].
The integrated PAM/OCT imaging system is composed of three main parts: (1) the PAM system, (2) the OCT system, and (3) a dichroic mirror and two-dimensional galvanometer for beam combining and scanning (Fig. 4A) [43,44]. In the PAM system, a tunable dye laser is used to excite the target optically, an unfocused needle transducer is used to detect PA waves, and a data acquisition board is used to digitize the data. The interference signal in the spectral domain is detected by a conventional spectral domain OCT system with a broadband laser source, spectrometer, and a line charge-coupled device camera. The dichroic mirror is used to combine the pulsed laser beam in the PAM system and the broadband laser beam in the OCT system. The two-dimensional galvanometer is used for raster scanning; it is synchronized with the data acquisition board and the OCT computer by reference to the external trigger signal from the PAM pulsed laser.
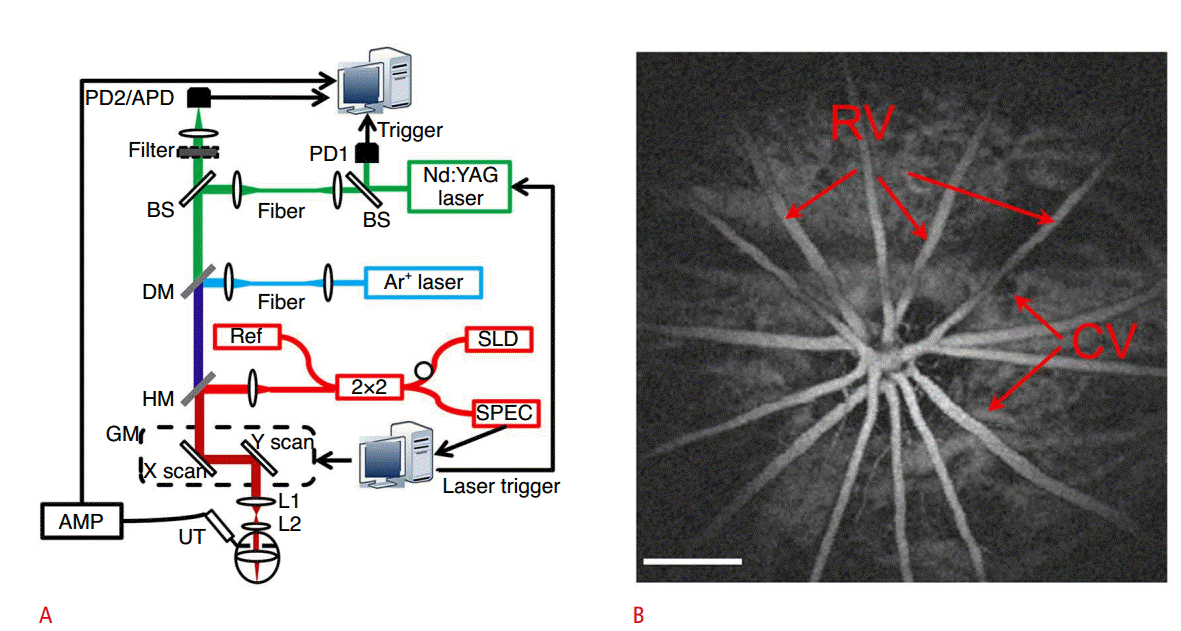
Experimental setup of PAM/OCT imaging system and example image.
A. A tunable dye laser excites the target, and an unfocused needle transducer detects PA waves. A conventional spectral domain OCT system with broadband laser source, spectrometer and a line CCD camera are used to detect the interference signal in the spectral domain. The dichroic mirror combines the pulsed laser beam in the PAM system with the broadband laser beam in the OCT system. The two-dimensional galvanometer is used for raster scanning. B. Maximum amplitude projection PAM image of retinal vasculatures. Scale bar=500 µm. PD, photodiode; APD, avalanche photodetector; BS, beam splitter; Nd:YAG, neodymium-doped yttrium aluminum garnet; DM, dichroic mirror; HM, hot mirror; SLD, super luminescent diode; SPEC, spectrometer; GM, two-dimensional galvanometer; AMP, amplifier; UT, ultrasound transducer; RV, retina vessels; CV, choroidal vessels; PAM, photoacoustic microscopy; OCT, optical coherence tomography; PA, photoacoustic. Reprinted from Song et al. J Biomed Opt 2012;17:061206 [43], with permission of SPIE.
B-scan images of the retinal vasculature and choroid vessels in a rat were obtained using an integrated PAM/OCT imaging system. The maximum amplitude projection PAM image of the retinal vessels was also acquired (Fig. 4B). The integrated PAM/OCT imaging system provides both structural and functional information. Using PAM, the retinal oxygen saturation value can be measured using multi-wavelength scanning. Furthermore, the OCT system can measure the blood flow velocity by exploiting the Doppler effect.
The integrated PAM/OCT system can be simplified by using one light source for both PAM and OCT. Optical coherence PAM uses a broadband neodymium-doped yttrium aluminum garnet pulsed laser source [45]: a 532-nm wavelength pulsed laser beam is used for PAM, and a laser beam with a 580-nm center wavelength and 20-nm bandwidth is used for OCT. Another method for simple implementation uses a noncontact US signal detection approach [46]. By replacing the piezoelectric US transducer with an interferometer, the PA signal can be detected without touching the transducer to the sample. The advantage of this approach is that the interferometer can be also used for OCT. Therefore, noncontact PAM and OCT imaging systems can be combined easily.
OCT is an imaging method that is based on optical scattering and is widely utilized to acquire micron-scale structural information about biological tissues. PA imaging can provide additional physiological information about target objects. Therefore, a comprehensive range of information can be obtained by combining OCT and PAM, including tissue anatomy, hemoglobin concentration, blood flow, and saturated oxygen values.
PAM and MPM
MPM uses a low-energy wavelength laser for FL imaging of living cells and microscopic objects [47,48]. MPM exploits the fact that the FL effect is confined to a volume of less than one picoliter; therefore, the system can acquire a high-resolution FL image without using a pinhole. In this case, a nonlinear effect can occur due to the very rare instances in which a molecule absorbs more than one photon simultaneously. MPM has the advantage that it causes less photobleaching than conventional FL microscopy. MPM works by stimulating a target that has been labeled using a fluorescent dye, fluorescent proteins, nanoparticles, or specific natural fluorescent materials. Therefore, the targeted imaging of specific molecules is possible. Functional activity information about living tissue can also be obtained.
MPM is used in the biomedical field for molecular imaging, neuroscience, and cancer detection. MPM provides molecular information, such as the levels of reactive oxygen species, glutamate, NADH, FAD, and oxygen. MPM can also visualize the structural and physiological properties of cells, complex neural circuits, nerve activities, and blood flow [49], and can provide fluorescent images in a deeper area than can existing FL microscopy. MPM achieves a wide wavelength-tuning range (690-1,050 nm) by using a titanium-sapphire laser. The PAM laser has also a wide wavelengthtuning range (350-750 nm) that overlaps with the range of the MPM system. These advantages enable the acquisition of various functional information for targeted imaging.
PAM and MPM are high-resolution imaging technologies that can provide structural and physiological information of cells at a depth level in the millimeter range [50-52]. The two techniques were developed separately, but have recently been combined to improve diagnosis and analysis at high contrast. Combined technologies, such as OCT/MPM, OCT/PAT, US/PA, and confocal/MPM, will combine the strengths of these technologies and compensate for their individual weaknesses [53,54].
Recently, a new integrated system has been developed, combining PAM, confocal microscopy, and MPM [25]. This means that MPM can be extended to new biological investigations by measuring nonfluorescent substances such as hemoglobin and melanin. In the combined system (Fig. 5A), two polarization beam splitters and a half-wave plate combine the optical source of each technology into an optical common path in a commercial microscope. The PA signals generated by the sample are detected by an acoustic transducer in the microscope body. Dichroic mirrors then split the backwardpropagating fluorescent light into two beams. One is sent to the confocal detector and one is sent to the MPM signal detector. Images of moss leaves were acquired: the MPM image visualized leaf cell boundaries and chloroplasts inside the leaf cells (Fig. 5B), a confocal image was obtained based on FL signals from the chloroplasts (Fig. 5C), and the optical resolution PAM maximum amplitude projection images visualized the moss leaves at optical wavelengths of 570 nm (Fig. 5D) and 578 nm (Fig. 5E). The maximum amplitude images of the MPM, confocal microscopy, and 570-nm optical-resolution PAM systems were merged into one overlay as green, red, and blue, respectively (Fig. 5F), and a differential absorption contrast image was generated in order to visualize the change in optical absorption across multiple wavelengths (Fig. 5G). The differential absorption contrast images can provide functional information about nonfluorescent chromophores.
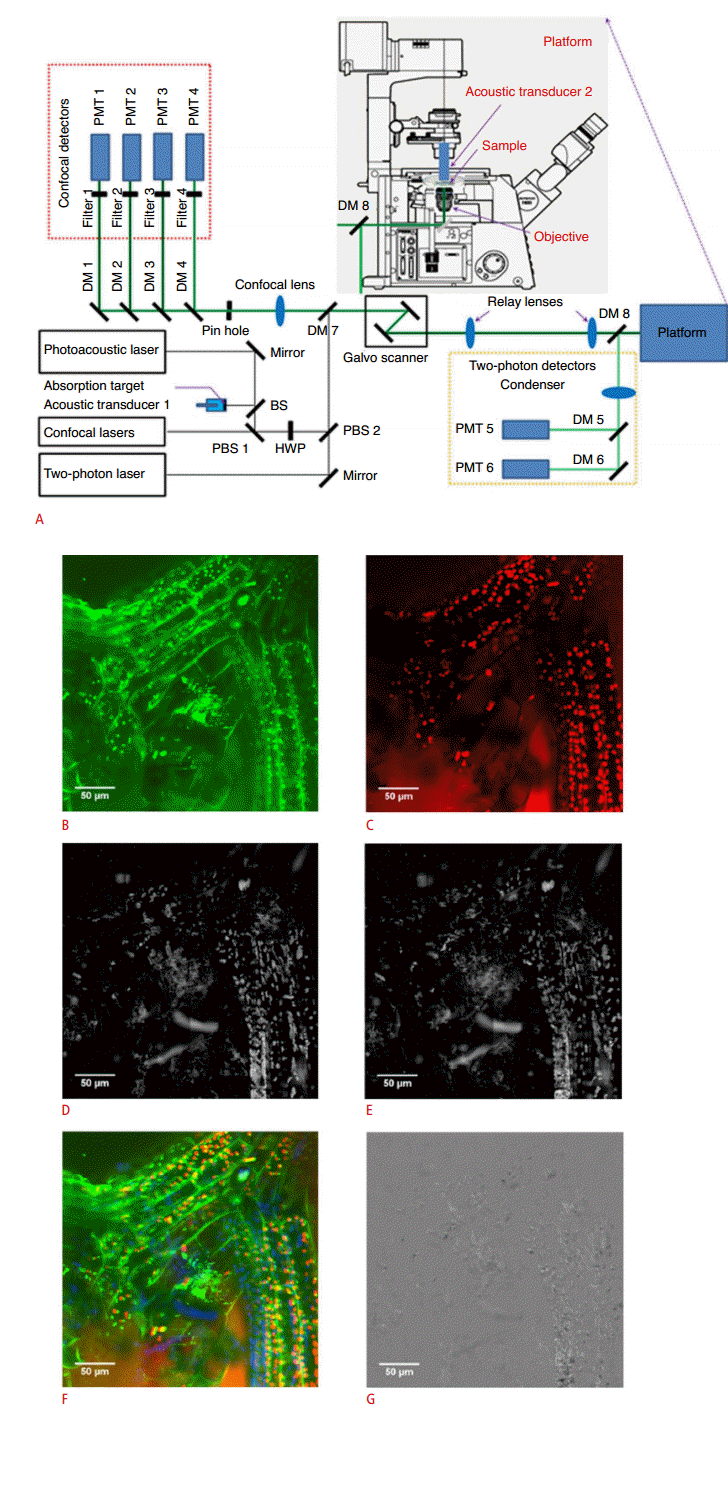
Experimental setup of the combined confocal, two-photon, and optical-resolution microscope, and example images.
A. Two polarization beam splitters and a half-wave plate combine the optical sources into a common optical path in a commercial microscope. The generated PA signals are detected by an acoustic transducer in the microscope. Then dichroic mirrors split the backwardpropagating fluorescent light into two beams; one is sent to the confocal detector, and the other is sent to the MPM signal detector. B. MPM image of leaf cell boundaries and chloroplasts. C. Confocal image of FL signals from chloroplasts. D. OR-PAM MAP image of moss leaves at 570 nm. E. OR-PAM MAP image of the moss leaves at 578 nm. F. Merged image rendered from MPM, confocal, and 570 nm OR-PAM image as green, red, and blue color respectively. G. Differential absorption contrast image of OR-PAM MAP image. PMT, photomultiplier tube; DM, dichroic mirror; BS, beam splitter; PBS, polarization beam splitter; HWP, half-wave plate; PA, photoacoustic; MPM, multi-photon microscopy; FL, fluorescence; OR-PAM, optical-resolution photoacoustic microscopy; MAP, maximum amplitude projection. Reprinted from Rao et al. J Biomed Opt 2014;19:36002 [25], with permission of SPIE.
Optical absorption and FL targets can be imaged simultaneously using combined PA and FL imaging systems. This approach can also be applied to microscopic imaging technologies. The beauty of dual-mode PAM and MPM is that the combined imaging system can show both fluorescent and non-fluorescent chromophores at a micron scale.
Summary
The multimodal capability of PA imaging techniques was reviewed. PA imaging is a noninvasive and nonionizing imaging method that is based on both optical absorption and the generation of acoustic waves by thermoelastic expansion. PA imaging is safe, fast, and inexpensive. A PA imaging system can be easily integrated with various imaging modalities such as US, FL, OCT, and MPM. The strengths of PA imaging complement the weaknesses of each of the above imaging methods. Furthermore, integrated systems can provide structural information (i.e., surrounding blood vessels, organs, and microstructures), physiological information (i.e., hemoglobin concentration, hemoglobin oxygen saturation, and angiogenesis), and molecular information (i.e., exogenous contrast agent uptake). PA imaging could also potentially be integrated with other biomedical imaging methods, including magnetic resonance imaging, computed tomography, positron emission tomography, and single photon emission computed tomography.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This work was supported by the research funds from the following grants: NRF-2013K1A3A1A20046921 (China-ROK Joint Research Project), NRF-2011-0030075 (Engineering Research Center), NRF-2014M3C1A3017229 (NRF Pioneer Research Center Program), and NIPA-2014-H0201-14-1001 (IT Consilience Creative Program of the Ministry of Science, ICT and Future Planning [MSIP], Republic of Korea).
