Ultrasonography of intrauterine devices
Article information
Abstract
The intrauterine device (IUD) is gaining popularity as a reversible form of contraception. Ultrasonography serves as first-line imaging for the evaluation of IUD position in patients with pelvic pain, abnormal bleeding, or absent retrieval strings. This review highlights the imaging of both properly positioned and malpositioned IUDs. The problems associated with malpositioned IUDs include expulsion, displacement, embedment, and perforation. Management considerations depend on the severity of the malposition and the presence or absence of symptoms. Three-dimensional ultrasonography has proven to be more sensitive in the evaluation of more subtle findings of malposition, particularly side-arm embedment. Familiarity with the ultrasonographic features of properly positioned and malpositioned IUDs is essential.
Introduction
First described for humans in 1909 by Dr. Richard Richter [1], the intrauterine device (IUD) is the most popular reversible form of contraception today, with more than 168 million users worldwide [2]. However, there is still large-scale regional variation in the use of IUDs. Eighty-three percent of IUD users worldwide live in Asia [3]. The use of IUDs in the United States has been traditionally much lower than in many European countries but is slowly increasing. The most recent statistics estimate that 5% of the contracepting women in the United States opt for IUD placement (up from 0.8% in 1995) [4]. Most IUDs are inserted without image guidance. Ultrasonography plays an essential role in evaluating IUD position and assessing for complications. This review focuses on the ultrasonography of IUDs and presents critical imaging features of properly and improperly positioned IUDs.
IUD Types and Placement
Both copper and hormone-releasing IUDs are currently available in the United States. The copper TCu-380A (ParaGard, Teva Women’s Health, Inc., North Wales, PA, USA) is made of a T-shaped polyethylene frame with barium sulfate added for radiopacity (Fig. 1A). Exposed copper on the arms and stem release copper ions, which both increase the local foreign body inflammatory response and interfere with sperm mobility and viability preventing fertilization [5]. Two polyethylene monofilaments connected to the stem, referred to as retrieval strings, allow for detection and removal. TCu-380A is approved for up to 10 years of use.
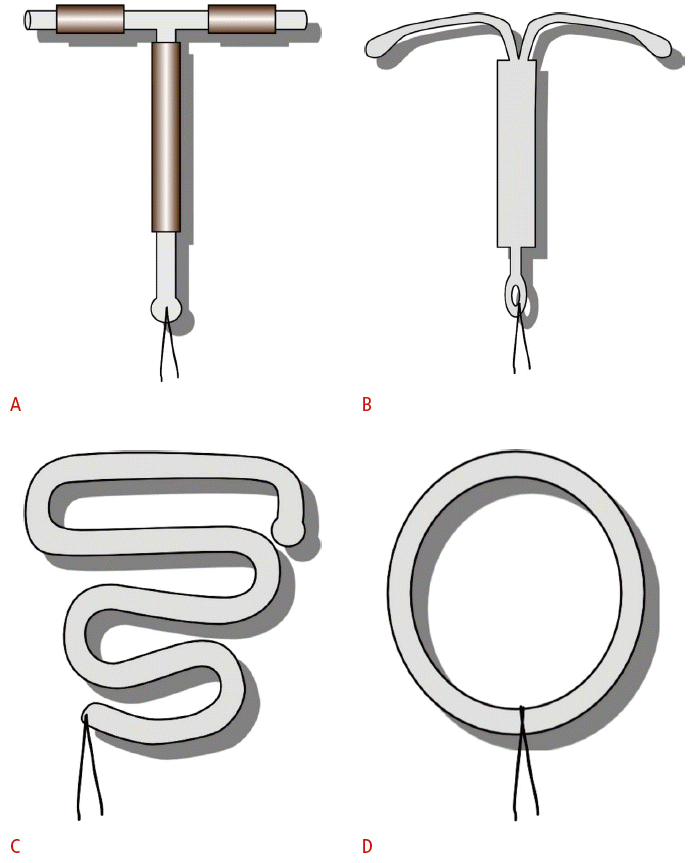
Schematic representation of intrauterine device (IUD) shapes.
A. T-shaped TCu-380A copper IUD exposes copper on both the stems and the arms. B. Levonorgestrel-releasing IUD is also T-shaped. C. Double “S”- shaped Lippes loop IUD was commonly used in the 1960s to 1980s. D. Stainless steel ring was used primarily in China before 1993.
The available hormone-releasing IUD in the United States is the intrauterine levonorgestrel-releasing system (Mirena, Bayer HealthCare Pharmaceuticals, Pittsburgh, PA, USA). It is also a radiopaque T-shaped device (Fig. 1B). Release of the embedded levonorgestrel, a synthetic progesterone, leads to cervical mucosal thickening and suppression of the endometrium as well as the inhibition of ovulation in some women [5]. It is approved for up to 5 years of use but has been shown to maintain efficacy for at least 7 years [6]. Because of the endometrial suppression, levonorgestrel-releasing IUDs are also approved to treat heavy menstrual bleeding in women using intrauterine contraception.
While this review focuses on the currently available T-shaped copper and hormone-releasing devices, inert IUDs such as the Lippes Loop (Ortho Pharmaceutical, Raritan, NJ, USA) (Fig. 1C) and stainless steel rings (Fig. 1D) can still be found in older patients. For example in China, stainless steel rings were popular before copper IUDs became preferred in 1994 [7].
Placement of IUDs is performed in an outpatient setting by using available kits and sterile technique. A sterile uterine sound is used to ensure a minimum uterine depth of 6 cm [8]. Image guidance is generally reserved for women with a history of difficult insertion, obesity that limits bimanual exam, or suspected distorted uterine cavity [9]. Follow-up pelvic examination within 6 weeks of insertion is recommended to ensure visualization of the retrieval strings, which should protrude through the external cervical os by 2-3 cm.
The correctly positioned IUD is located in the uterine cavity near the fundus (Fig. 2). The stem should extend toward the cervix and the two arms should be fully unfolded during insertion, reaching laterally toward the uterine cornua.
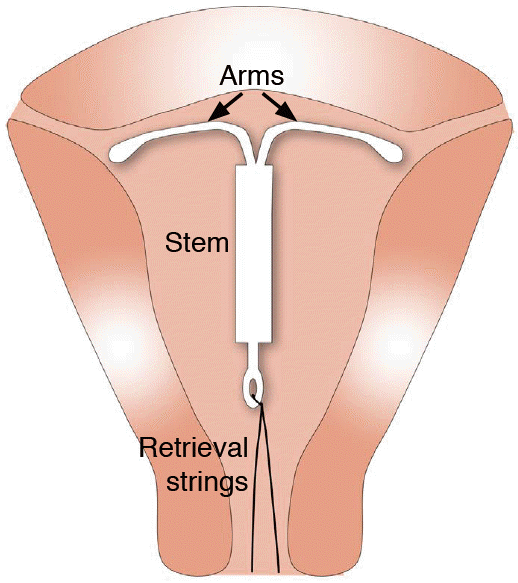
Schematic representation of normal intrauterine device (IUD) position.
The correctly positioned IUD is shown within the uterine cavity near the fundus. The two arms are fully unfolded, reaching laterally toward the uterine cornua. The stem extends inferiorly with the retrieval strings exiting through the cervix.
Imaging of IUDs
Imaging plays a crucial role in the management of patients with IUDs. Ultrasonography is the most common initial method of evaluation due to its cost-effectiveness, lack of ionizing radiation, and greater detail of pelvic anatomy [10]. The stem is usually easily identified on standard two-dimensional (2D) transvaginal ultrasonography (TVUS) as a linear echogenic structure (Fig. 3A-D). While the arms of the copper IUD are also fully echogenic, the arms of the levonorgestrel-releasing IUD are only echogenic at the proximal and distal ends, with characteristic central posterior acoustic shadowing on transverse images (Fig. 3D) [11]. Three-dimensional (3D) reconstructions are increasingly being used, particularly in the coronal view, which allows for a more careful evaluation of the arm positioning (Fig. 3E) [12,13]. In one study, all 28 cases of side-arm embedment into the myometrium could only be detected on the 3D coronal view [14].

Transvaginal ultrasonographic appearance of T-shaped intrauterine devices (IUDs).
A, B. Two-dimensional (2D) sagittal (A) and transverse (B) sonograms show hyperechoic levonorgestrel-releasing IUD in the endometrial cavity. C, D. 2D sagittal (C) and transverse (D) sonograms show the bright echo of the copper IUD with marked posterior shadowing. E. Three-dimensional coronal reformatted sonogram demonstrates the properly positioned copper IUD within the endometrial cavity (arrows).
Other imaging modalities can be accessory in select cases. When the IUD cannot be seen on pelvic ultrasonography, abdominal radiographs can be used to evaluate IUD positioning, as all IUDs are radiopaque. Positioning on an abdominal radiograph varies with normal uterine positions, but the IUD should be located near the midline low in the pelvis and orientated with the arms superior to the stem (Fig. 4A, B). In cases where complications such as perforations or abscesses are suspected, computed tomography (CT) or magnetic resonance imaging (MRI) may be a helpful adjunctive modality given their larger field of view. However, the associated radiation with CT and the cost of MRI limits their utility as a first-line modality for the evaluation of IUD position. Of note, both copper and hormone-releasing devices are considered safe for up to 3-T MRI [15]. Stainless steel IUDs have not undergone testing. If an IUD is present on CT or MRI performed for indications other than the assessment of the IUD itself, it is important for the radiologist to evaluate for its proper position (Fig. 4C, D).
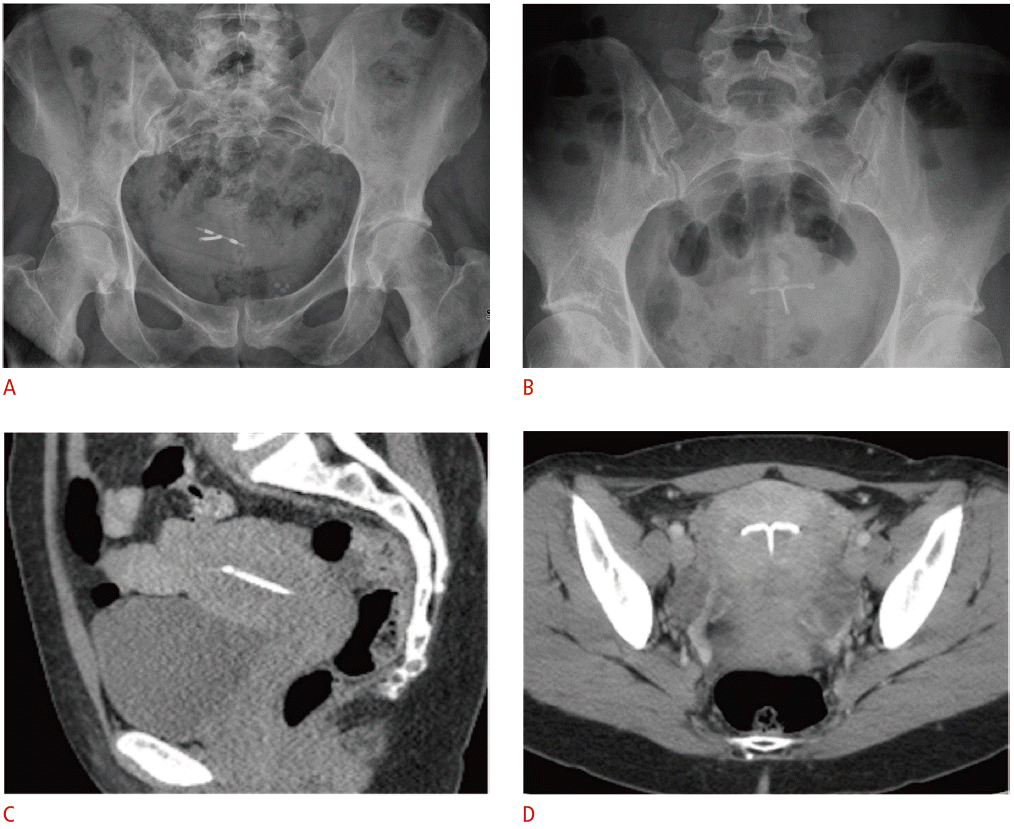
Radiographic and computed tomographic (CT) appearance of the T-shaped intrauterine device (IUD).
A, B. For the copper IUD in a retroverted uterus (A) and levonorgestrel-releasing IUD in an anteverted uterus (B), pelvic radiographs alone are inadequate for precise localization in relation to the uterine cavity due to normal variations in uterine position. C, D. Coronal (C) and sagittal (D) noncontrast-enhanced CT images demonstrate a radiodense IUD properly positioned within the uterine fundus.
Malpositioned IUD
The malpositioned IUD can be considered a spectrum of abnormal positioning (Table 1). At one end of the spectrum is complete ‘expulsion’ through the external cervical os. At the other end is complete ‘perforation’ through the uterine serosa with migration of the device into the intraperitoneal space. Along the spectrum is ‘displacement’ from the proper positioning within the fundus into the lower uterine segment or cervix and ‘embedment’ of a portion of the stem or arms into the myometrium without penetration of the serosa. These descriptions are non-exclusive. For instance, a displaced IUD may also be partially embedded. In one retrospective study of ultrasonography for any indication in patients with IUDs, almost 11% were malpositioned [16]. Malposition is more often associated with symptoms of pain and excess bleeding but can also be asymptomatic [14]. It is suspected clinically when there is shortening, lengthening, or absence of retrieval strings on pelvic exam [17].
Expulsion
The expelled IUD has passed inferiorly, either partially or completely through the external cervical os. The expulsion risk is greatest in the first year of use and the expulsion rate is highest with immediate postpartum placement after vaginal delivery [18]. A large retrospective study showed first-year expulsion rates of approximately 6% with TCu280A and 3% with Mirena [19]. When the expelled device has been identified or is seen on physical exam, expulsion can be managed without imaging. However, in cases of absent retrieval strings without witness of the expelled device, pelvic ultrasonography should be performed to evaluate the IUD position. If no IUD is identified by ultrasonography, an abdominal radiograph is recommended to exclude perforation and intraperitoneal migration. Management of the partially expelled IUD includes removal with a pair of alligator forceps or an IUD hook, generally in an outpatient setting.
Displacement
Displacement refers to any IUD that is rotated from the normal transverse position or located away from the fundus and within the lower uterine segment or cervix (Fig. 5). Early studies defined displacement as a distance of more than 3 mm between the IUD and the uterine fundus, which was initially thought to be associated with a high risk of expulsion [20]. However, more recent studies have shown that a majority of low IUDs move to a fundal position within a few months [21].
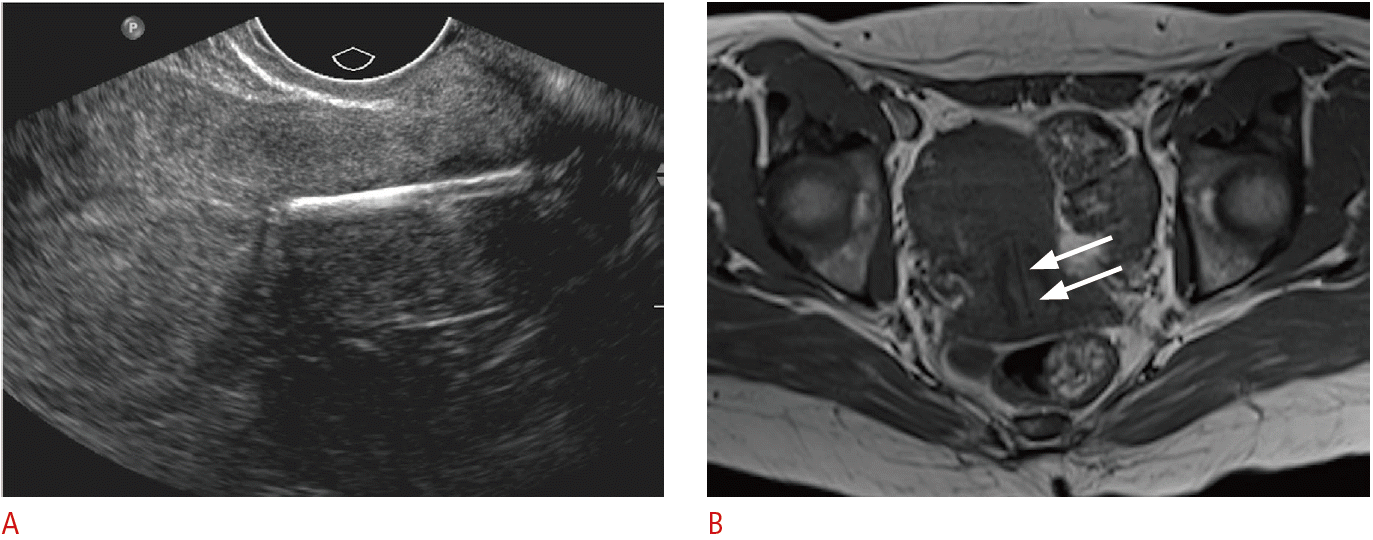
Incidentally detected displaced intrauterine device (IUD) in a 38-year-old female.
A. Sagittal transvaginal sonogram demonstrates the echogenic IUD stem within the cervix. B. Axial T1-weighted magnetic resonance image shows the low-signal IUD stem within the cervix (arrows).
The question of efficacy guides the management of the displaced IUD. Although no formal analysis has been performed at our institution, we have experienced both isolated cases of early intrauterine pregnancy (Figs. 6, 7) and ruptured ectopic pregnancy (Fig. 8) with displaced cervical IUDs. A displaced copper IUD has decreased efficacy; however, a displaced hormone-releasing IUD is equally effective as a properly positioned one [22]. A case control study showed patients who became pregnant with a copper IUD had significantly increased rates of malposition as compared to control nonpregnant copper IUD patients (64% vs. 11%) [23]. Any decreased efficacy of a displaced IUD must be weighed against the very real risk of pregnancy if the displaced IUD is removed without the implementation of an alternative form of contraception. A recent retrospective study reported a two-fold increased pregnancy rate in patients with malpositioned IUDs, but all resulted from removed IUDs. No pregnancies occurred with a displaced IUD in situ [16]. Because of this risk and the differences in efficacy, some researchers advocate for the removal of displaced copper IUDs but not hormone-releasing devices in asymptomatic patients [9]. Regardless, findings of a malpositioned IUD should be communicated to the referring physician.
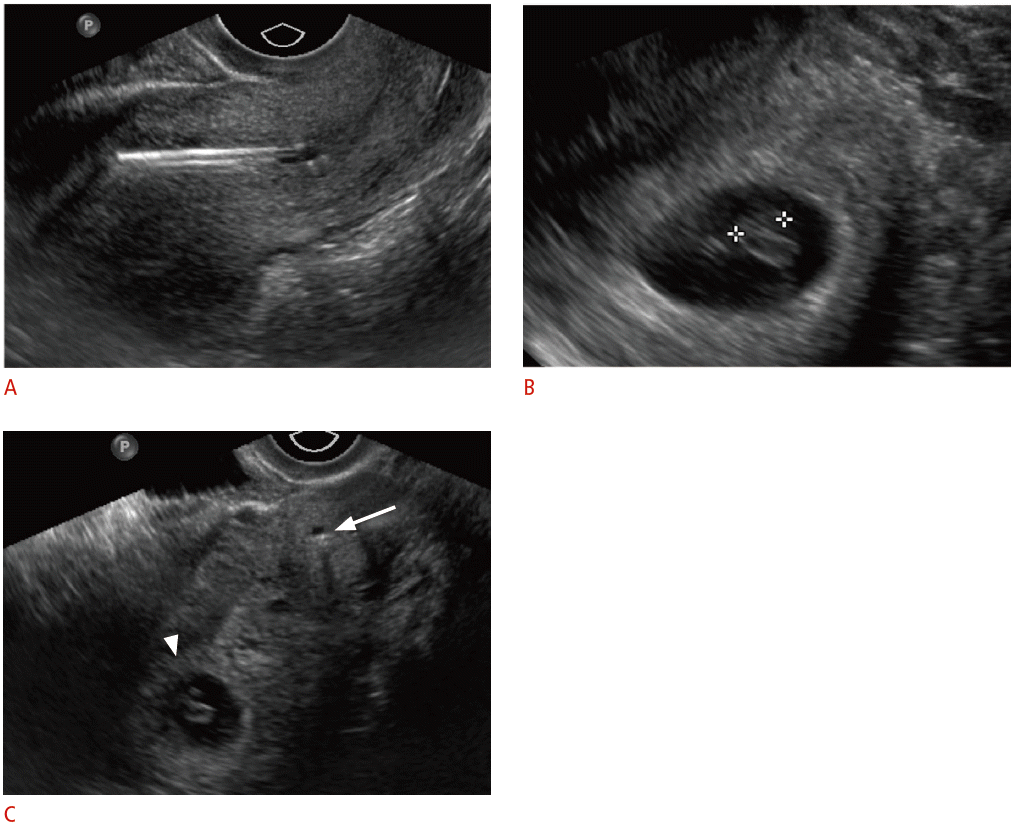
Displaced intrauterine device (IUD) in a 23-year-old female with positive pregnancy test despite IUD.
A. Sagittal transvaginal sonogram shows malpositioned IUD within the lower uterine segment and cervix. B. An intrauterine pregnancy is seen within the uterine fundus. C. Transverse transvaginal sonogram shows the relationship between the low-lying IUD within the cervix (arrow) and the gestational sac within the uterine fundus (arrowhead). The IUD was removed without incident. The pregnancy resulted in a normal, full-term delivery without adverse complications.
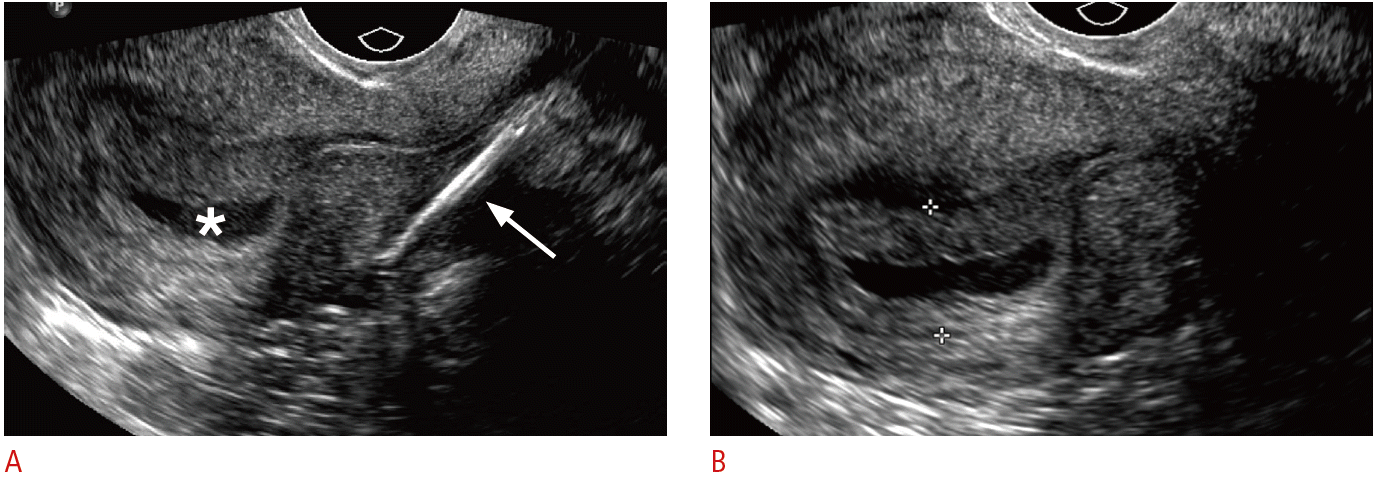
Displaced and embedded intrauterine device (IUD) with early pregnancy in a 24-year-old female with acute abdominal pain and positive pregnancy test.
A. Sagittal transvaginal sonogram shows the IUD stem displaced within the lower uterine segment and embedded in the posterior myometrium (arrow) and a gestational sac in the uterine fundus (asterisk). B. Zoomed in transvaginal sonogram of the gestational sac clearly shows a double decidual sac sign (between crosshairs). Rising human chorionic gonadotropin (β-HCG) was consistent with pregnancy, although the outcome of the pregnancy is unknown.
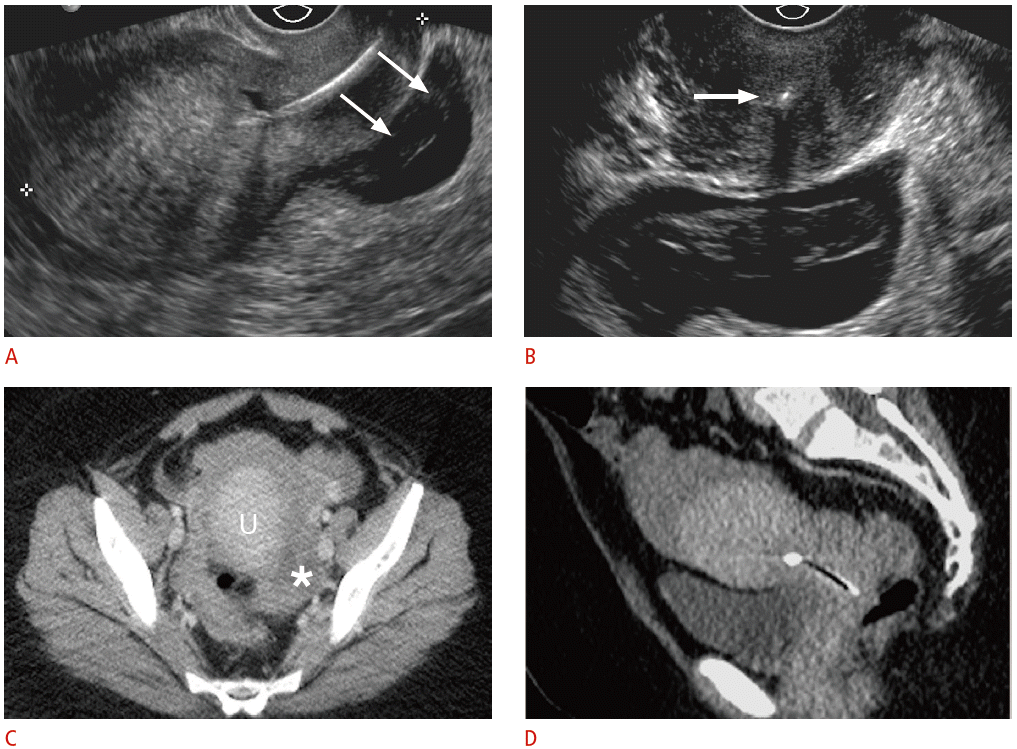
Displaced intrauterine device (IUD) with ruptured ectopic pregnancy in a 33-year-old female having acute pelvic pain.
A. Sagittal transvaginal sonogram shows the IUD positioned almost entirely within the cervix with a complex fluid collection posterior to the cervix (arrows). B. Transverse transvaginal sonogram demonstrates internal complexity within the fluid collection, posterior to the IUD positioned within the cervix (arrow). The left ovary was not identified, and computed tomography (CT) was recommended for further evaluation. The pregnancy status was not known at the time. C, D. Axial (C) and (D) sagittal CT show a large amount of hemoperitoneum surrounding the uterus (U) and a complex structure in the left adnexa (asterisk). Human chorionic gonadotropin (β-HCG) level was 595 mIU/mL (normal, <3.0 mIU/mL), and the patient underwent emergent laparoscopy for ruptured tubal ectopic pregnancy.
Embedment
Embedment refers to the penetration of the myometrium by the arm or stem of the IUD without extension through the serosa. When involving the stem, this may be obvious on standard 2D TVUS, but in cases of more subtle arm embedment, 3D coronal images allow for better detection (Figs. 9, 10). With the added sensitivity of 3D techniques, the incidence of embedment was found to be as high as 16.8% [13]. Extension into the myometrium is thought to occur at the time of insertion. When these findings are associated with symptoms of pain or abnormal bleeding, IUD removal is recommended [9]. Often, embedment occurs in combination with displacement, which also leads to the same management decisions based on efficacy as those previously discussed (Fig. 7).
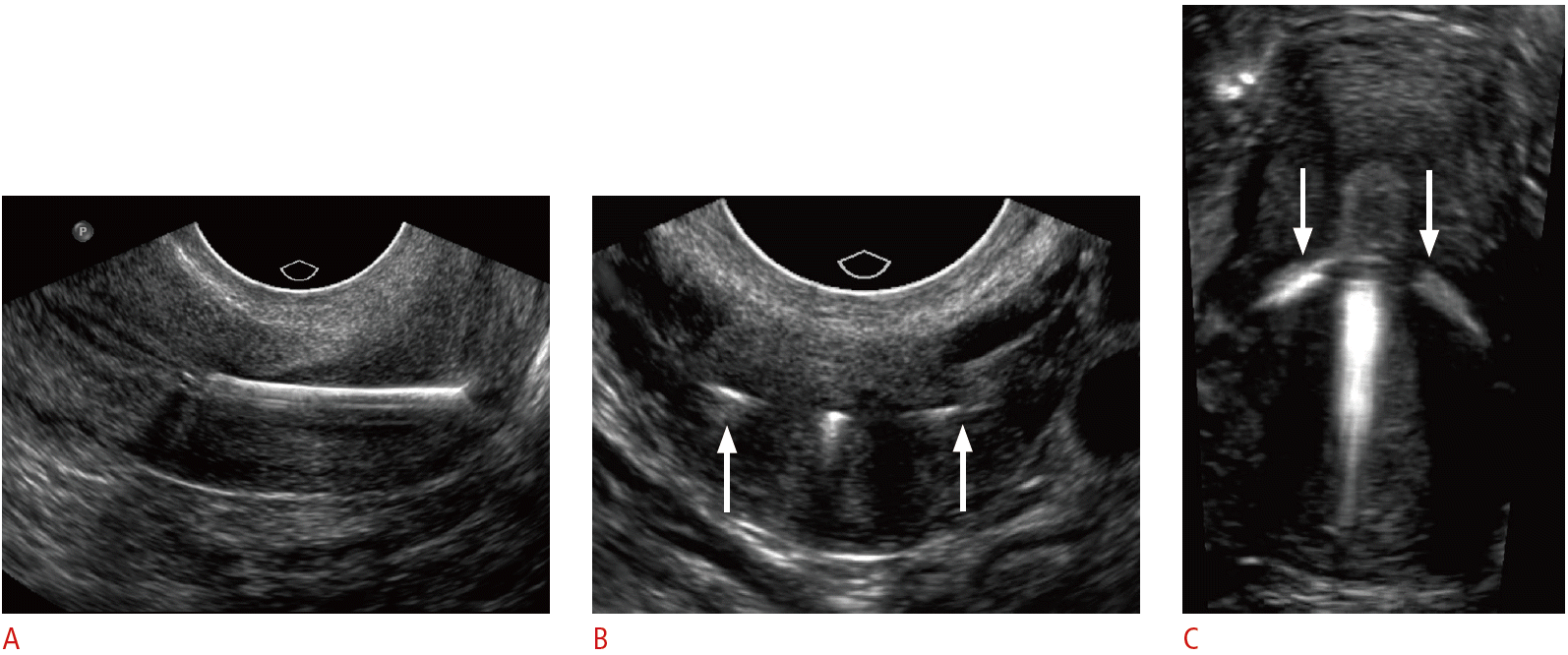
Embedded intrauterine device (IUD) in a 24-year-old female with menorrhagia and unsuccessful attempts at IUD removal.
A. Sagittal transvaginal sonogram shows inferior displacement of a copper IUD within the cervix. B. Transverse transvaginal sonogram of the cervix shows the arms of the IUD extending into the cervical wall (arrows). C. Three-dimensional coronal sonogram better demonstrates both arms embedded within the myometrium (arrows). The IUD was subsequently removed under anesthesia.
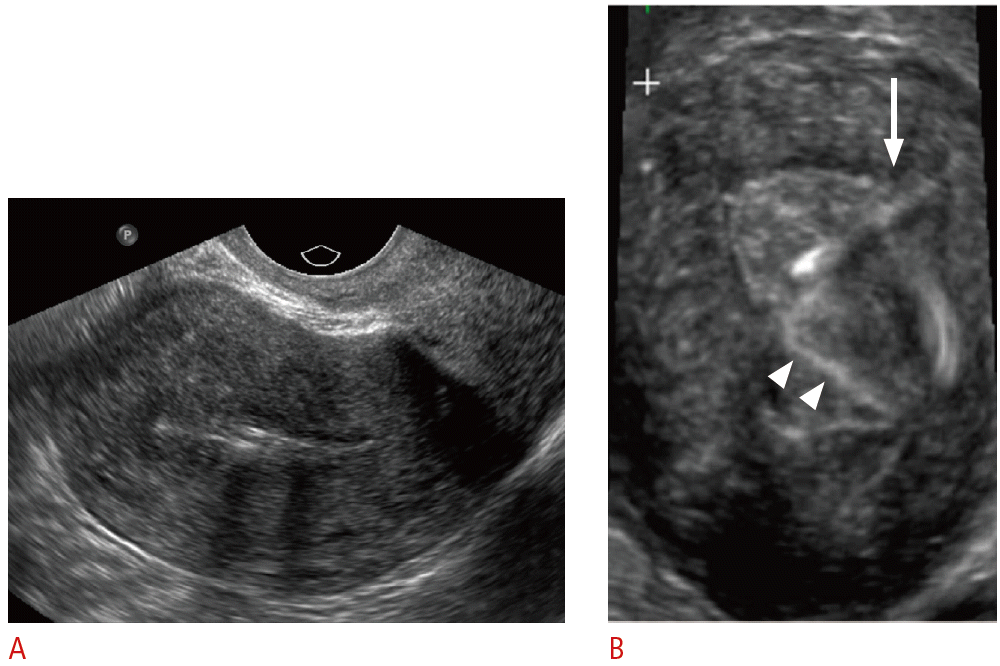
Embedded intrauterine device (IUD) in a 31-year-old female presents with the absence of retrieval strings.
A. Sagittal transvaginal sonogram shows the IUD within the endometrial canal. B. Three-dimensional coronal sonogram more clearly demonstrates the IUD obliquely tilted and embedded in the left uterine myometrium (arrow). The echogenic retrieval string is seen within the endometrial canal (arrowheads).
Perforation
The perforated IUD may penetrate through the serosa, either partially (Fig. 11) or completely with migration into the intraperitoneal cavity (Fig. 12). As with embedment, perforation occurs at the time of insertion. Perforation through the serosa occurs in one to two cases per 1,000 and is more often seen with inexperienced operators, with early postpartum placement, and in women with either few prior pregnancies or multiple miscarriages [24].

Partially perforated intrauterine device (IUD) in a 43-year-old female with shortened retrieval strings.
A. Sagittal transvaginal sonogram shows the stem extending through the myometrium of the posterior wall. B. Transverse transvaginal sonogram shows the arms extending outside the serosa (arrows). C, D. Midline (C) and left lateral (D) sagittal magnetic resonance images demonstrate correlated findings of the low-signal IUD coursing through the posterior myometrium (arrowhead) with the arms extending through the serosa (arrows).
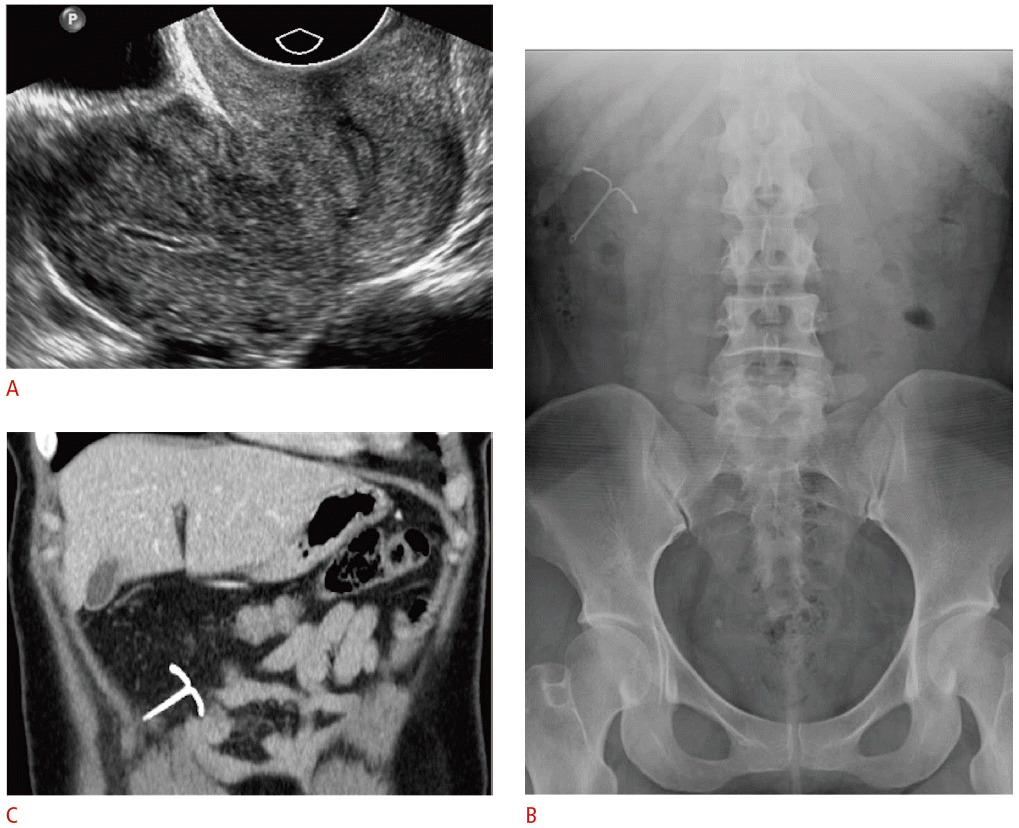
Complete intrauterine device (IUD) perforation with intraperitoneal migration in a 30-year-old female with absent retrieval strings and right upper-quadrant abdominal pain.
A. Transvaginal ultrasonography was unable to identify an IUD within the uterus or cervix. B. Frontal radiograph of the abdomen and pelvis shows the IUD in the right upper-quadrant. C. Contrast-enhanced computed tomography (CT) was ordered to assess for bowel injury given the patient’s abdominal pain. Coronal CT image demonstrates the IUD within the omentum without evidence of bowel injury. The IUD was removed laparoscopically and was found to be entangled within the greater omentum.
Adhesions that form as a result of a foreign body reaction to the perforated IUD can involve the fallopian tubes and result in decreased fertility. Cases of complete perforation can also rarely be associated with injury to adjacent structures, most often the bowel. If an IUD cannot be identified on initial ultrasonography, abdominal radiographs are required to locate the IUD. Cross-sectional imaging can be used for surgical planning and to evaluate for complications such as abscess formation or bowel injury. Management includes surgical removal, which can usually be done laparoscopically.
Uncommon IUD Complications and Mimics
Fragmentation
IUDs may, rarely, be broken during expulsion or removal, including embedded retrieval strings (Fig. 13). Few data are available on the long-term effects of retained strings or pieces of devices and no clear management guidelines have been established [25]. Manual vacuum aspiration may be helpful in IUD removal in cases of embedment; otherwise, surgery may be required.
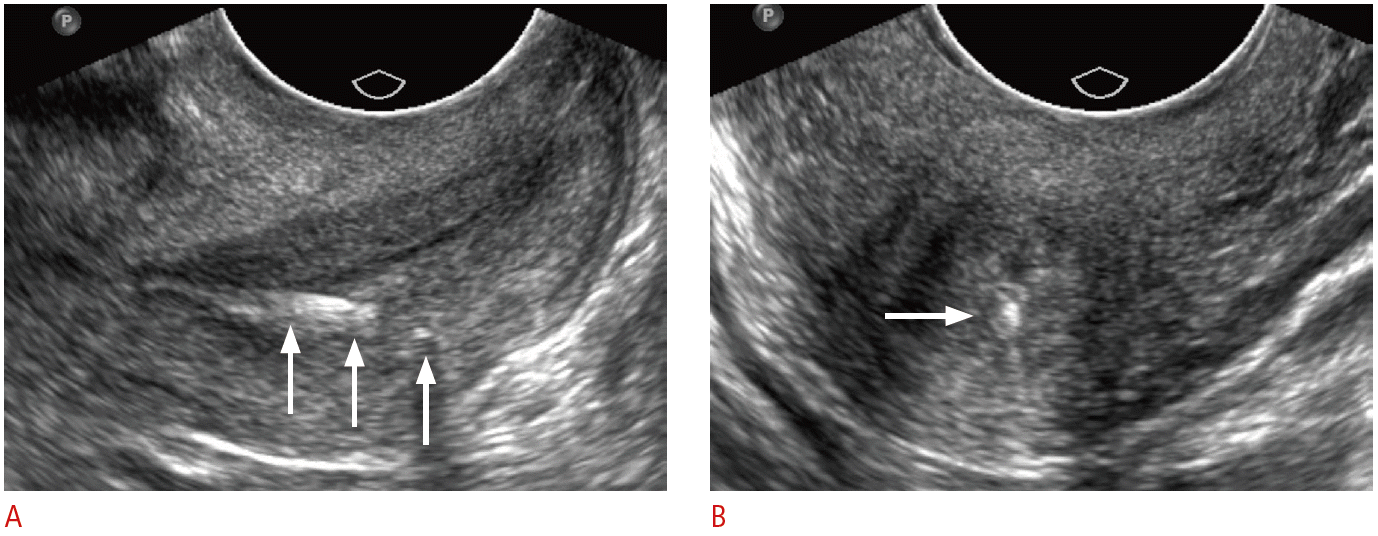
Retained intrauterine device (IUD) retrieval string in a 24-year-old female with crampy pelvic pain and history of IUD removal approximately a year ago.
Sagittal (A) and transverse (B) transvaginal ultrasonography of the cervix demonstrates a partially fragmented, linear echogenic structure (arrows) along the posterior wall of the cervix. The patient had a history of removal of an embedded IUD with portions of the retrieval strings left behind.
Calcification
Incrustation, the formation of calcium carbonate deposits on or near the IUD, is a well-described phenomenon that can be demonstrated as uneven echoes surrounding the normal IUD echoes (Fig. 14). The clinical significance of these calcifications is unclear. Early concern over associated inflammatory complications [26] have not been further investigated.
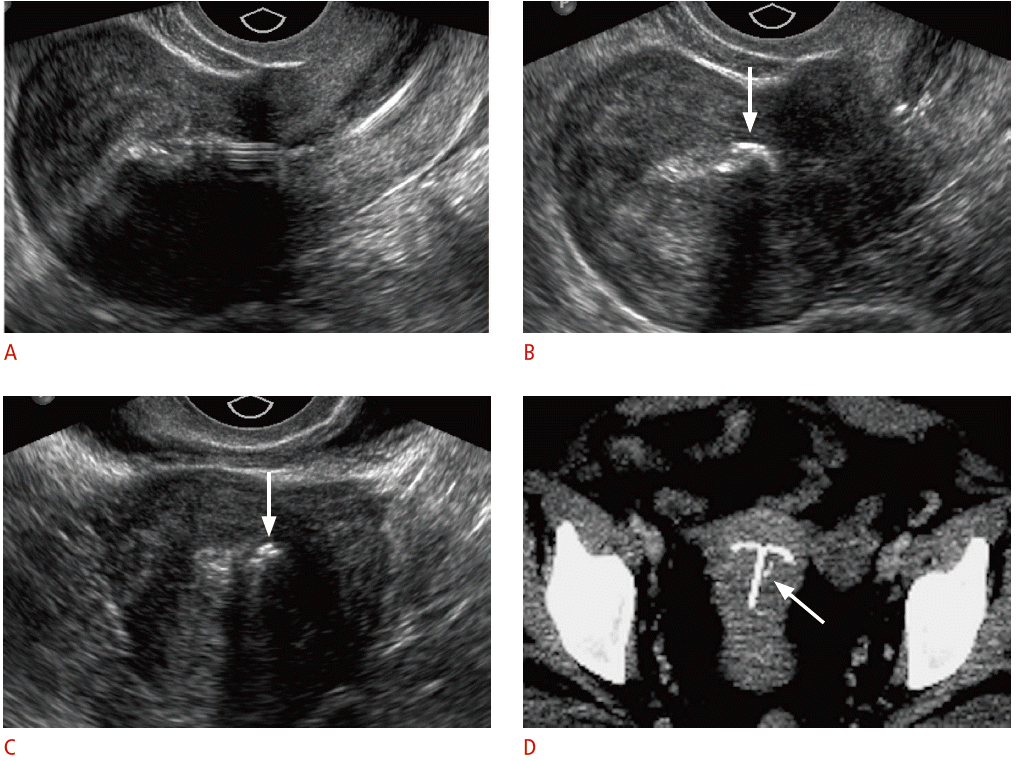
Calcifications associated with the intrauterine device (IUD) in a 39-year-old female with pelvic pain and a history of a levonorgestrel-releasing IUD placed approximately a year ago.
A. Sagittal transvaginal sonogram shows an IUD properly positioned midline within the uterine cavity. B, C. Sagittal (B) and transverse (C) transvaginal sonogram shows a coarse, echogenic shadowing structure (arrows) within the endometrial cavity adjacent to the IUD. D. The calcification (arrow) is seen faintly near the T-shaped IUD on the axial maximum intensity projection reconstruction computed tomography.
Mimics
Occasionally, linear echogenic intrauterine structures can be mistaken for an IUD. Retained fetal bone fragments are rare sequelae of spontaneous or induced abortions. Ultrasonography findings include linear or angular echogenic shadowing structures within the uterine cavity or myometrium [27]. In the absence of a clear history, this finding may be misinterpreted as an IUD on ultrasonography [28]. In fact, the retained fetal part is thought to act like an IUD, causing secondary infertility, which may be the only presenting symptom. Endometrial osseous metaplasia is a related and equally rare phenomenon, with the development of a mature bone in the endometrium, whose pathogenesis is controversial. Prevailing theories include development from retained fetal bone or true metaplasia of the endometrial tissue secondary to chronic inflammation. This can also be the cause of secondary infertility and be mistaken for an IUD [29].
Summary
As the IUD gains in popularity as a contraceptive device, it is becoming increasingly important for the referring gynecologist and radiologist to be informed of the characteristic ultrasonographic imaging features of positioned and malpositioned IUDs. In particular, the 3D coronal view is crucial in assessing for IUD displacement or an embedded IUD arm or stem within the myometrium. Radiography and CT scan imaging are helpful in confirming expulsion or assessing for perforation, intraperitoneal migration, and complications such as abscess or bowel injury. Ultrasonography is also helpful in the management of complications such as contraceptive failure (pregnancy) and detection of fragmentation and calcification.
Notes
No potential conflict of interest relevant to this article was reported.

