Ultrasonography of acute retroperitoneum
-
Hung-Hsien Liu1
 , Yenpo Lin2
, Yenpo Lin2 , Gigin Lin2,3
, Gigin Lin2,3 , Li-Jen Wang2,3
, Li-Jen Wang2,3 , Yung-Liang Wan2,3
, Yung-Liang Wan2,3
- Received December 18, 2023 Revised February 6, 2024 Accepted February 14, 2024
- ABSTRACT
-
The retroperitoneum is an important space in the human body that is often implicated in a range Epub ahead of print of acute medical conditions, some of which can be life-threatening. Ultrasonography may serve as a pivotal first-line imaging technique when assessing patients with suspected retroperitoneal abnormalities. Effective ultrasonography of the retroperitoneum requires a comprehensive grasp of its anatomy, adjacent structures, and potential pathologies. Being well-acquainted with the imaging characteristics of acute conditions can meaningfully assist in an accurate diagnosis and guide subsequent management. This review article summarizes and illustrates the acute conditions involving the retroperitoneum through the lens of ultrasound imaging.
- Key point
-
Key pointDisease in the retroperitoneum may present as acute conditions, some of which can be life-threatening. Ultrasonography may serve as a pivotal first-line imaging modality for patients with acute retroperitoneum. Effective ultrasonography of the retroperitoneum requires a comprehensive grasp of its anatomy, scanning techniques, and potential pathologies.
- Introduction
- Introduction
The retroperitoneum can be segmented into three spaces—namely, the anterior pararenal space, the perirenal space, and the posterior pararenal space. The anterior pararenal space is demarcated anteriorly by the peritoneum and posteriorly by the anterior renal fascia. The perirenal space is bordered by the anterior and posterior renal fascia, which fuse to become the latero-conal fascia, while the posterior renal space is delineated by the posterior renal fascia and transversalis fascia. The anterior pararenal space contains the pancreas, second and third portions of the duodenum, the vertical colon, and the distal common bile duct. The kidneys, adrenal glands, and ureters are located in the perirenal space. The posterior pararenal space lacks any primary visceral organs but contains soft tissue and vessels, mainly the aorta, inferior vena cava, and iliac vessels.Lesions in the retroperitoneum may present as acute abdomen, characterized by pain in the abdominal region, including the epigastric, flank, or back areas. The clinical presentations may be ambiguous and sometimes lead to a delayed diagnosis. As an imaging evaluation, ultrasonography provides the advantages of non-irradiation, lower cost, multiplanar and repeated scanning, and the availability of color flow information. With the accessibility of hand-held devices, practitioners are able to directly assess areas of patient discomfort at the bedside. This review aims to elucidate the distinct ultrasound characteristics associated with acute retroperitoneal conditions.
- Urolithiasis
- Urolithiasis
Urolithiasis often manifests as acute renal colic, with a classical presentation of acute onset and severe unilateral flank pain radiating to the groin area. Notably, approximately 30% of these individuals will also exhibit hematuria [1]. This condition is among the frequent reasons for emergency room visits. Multiple predisposing factors have been reported, such as diabetes mellitus, gout, obesity, a high-protein diet, and living in a warmer climate [2]. Ultrasonography and computed tomography (CT) of the abdomen and pelvis without contrast are commonly employed for confirmation of stones. On ultrasonography, urolithiasis typically appears as hyperechoic foci with an acoustic shadow. Hydronephrosis is another frequent observation. The size and location of the stones in the ureter are important factors for stone detection. Ultrasonography is highly effective when a ureter stone is larger than 5 mm [3]. However, it is often more difficult to detect smaller stones or when the stones are located in the middle third of the ureter due to obscuration by bowel gas and pelvic bone [4]. On ultrasonography, a dilated ureter may be identified as an anechoic tubular structure entering the urinary bladder, and hyperechoic foci with acoustic shadow may be found (Fig. 1). Color Doppler ultrasonography is helpful in delineating the sudden burst of color in the bladder lasting for a few seconds (Fig. 2). The thickness of the ureteral wall, which can be measured by ultrasonography, is associated with the likelihood of spontaneous stone passage. If the ureteral wall thickness exceeds 2.4 mm, it is less likely to have spontaneous stone passage with a sensitivity of 83% and a specificity of 86% [5]. Ultrasonography is the diagnostic imaging method of choice, especially for pregnant patients, due to its non-invasive nature and absence of radiation exposure. Additionally, considering the recurrent nature of urolithiasis, ultrasonography is favorable over CT scans to minimize cumulative radiation exposure. A randomized, multicenter trial by Smith-Bindman et al. showed that ultrasonography matched the diagnostic accuracy of CT scans but at a reduced cost [6]. They concluded that ultrasonography should be used as the initial diagnostic imaging test. The European Association of Urology 2016 Guidelines also recommended using ultrasonography as the primary diagnostic imaging tool before performing a non-enhanced CT scan in cases of suspected urolithiasis [7].
- Acute Pyelonephritis
- Acute Pyelonephritis
Acute pyelonephritis refers to a bacterial infection of the kidneys, stemming from either an ascending infection of the urinary tract or hematogenous spread. The diagnosis can be made from clinical symptoms of fever, flank pain, pyuria, and leukocytosis. While imaging often is not essential, it might be deemed necessary for severely ill patients or those unresponsive to treatments [8]. On ultrasonography, kidneys with acute pyelonephritis usually appear normal. The sonographic features of acute pyelonephritis, if present, are renal enlargement, decreased echogenicity, loss of renal sinus fat, and loss of corticomedullary differentiation (Figs. 3, 4) [9]. In cases where the infection is localized, a mass may be found [10]. Furthermore, ultrasonography can detect predisposing factors beyond abnormalities in the renal parenchyma, such as urolithiasis or other causes of urinary tract obstruction, and detect the severity of disease, such as gas-forming infections, abscesses, or hemorrhage [8,11]. These findings can guide the need for interventions necessary for infection control.
- Renal and Perinephric Abscesses
- Renal and Perinephric Abscesses
Severe acute pyelonephritis may cause parenchymal necrosis and abscess formation. The risk factors for renal and perinephric abscesses include urolithiasis, diabetes mellitus, and chronic kidney disease [12,13]. Common causative pathogens are Escherichia coli, Staphylococcus aureus, and Klebsiella species [14]. On ultrasonography, renal abscesses may appear as round, thick-walled, hypoechoic, or complex masses (Fig. 5). Internal septations and mobile debris may be seen. The accumulation of suppurative materials in the obstructed collecting system may lead to pyonephrosis or pyoureter, which can feature echogenic materials or fluid-fluid layering in the urinary tract (Fig. 6).The perinephric space is the central compartment of the retroperitoneum, which contains the kidneys, proximal ureters, and adrenal glands. Small perforating vessels contribute to a rich blood supply into the perinephric space, providing a hematogenous route for infection to spread. Hence, perinephric abscesses most frequently arise from preexisting renal abscesses due to urinary tract infections [15]. The risk factors, common pathogens, and ultrasonography features are similar to those of renal abscesses.
- Emphysematous Pyelonephritis
- Emphysematous Pyelonephritis
Emphysematous pyelonephritis is a rare form of severe bacterial renal infection, marked by gas formation within the inflammatory tissue. Ultrasonography may show an enlarged kidney without or with fluid collection within the renal parenchyma, collecting system, or perirenal area. Dirty echogenic foci with reverberation or ring-down artifacts representing gas may also be seen. Severe renal destruction with surrounding gas collection may feature curvilinear high echogenicity with a dirty shadow [16]. A report analyzed 38 patients with emphysematous pyelonephritis and categorized them into two types: type I and type II [16]. Type I emphysematous pyelonephritis is characterized by parenchymal destruction and streaky or mottled gas on radiographs or CT images (Fig. 7). This type is thought to suggest a poor immune response, hence a poor prognosis. On the contrary, type II emphysematous pyelonephritis is characterized as renal or perirenal fluid collections, with bubbly or loculated gas or with gas in the collecting system (Figs. 8, 9). The fluid collections are considered to reflect a favorable immune response and a better outcome [16]. Types I and II emphysematous pyelonephritis have a mortality rate of 68.8% and 18.0%, respectively. In cases of death, the clinical course is more fulminant in the former; the average number of days from onset to death was 10.5 days in type I emphysematous pyelonephritis, which was significantly more fulminant than the interval of 34.8 days in type II emphysematous pyelonephritis [16]. It has been reported that in cases of gas-producing renal infection, the poor prognosticators include radiological type I emphysematous pyelonephritis, impaired renal function, thrombocytopenia, and higher urinary red blood cell count, and a multivariate analysis revealed that an elevated serum creatinine level was the most significant prognosticator [17].
- Acute Pancreatitis
- Acute Pancreatitis
On ultrasonography, the normal pancreatic parenchyma appears isoechoic or hyperechoic compared with the hepatic parenchyma, and the echogenicity increases with age due to fatty replacement [18,19]. Acute pancreatitis, characterized by inflammation of the pancreas, often presents as intense pain in the upper abdomen. Laboratory tests typically reveal elevated levels of serum amylase and lipase. When acute pancreatitis is suspected within the initial 48–72 hours of onset, the American College of Radiology guidelines recommend ultrasonography as the primary and most suitable imaging modality [20]. However, during the early stages or in mild cases of the condition, the ultrasound findings may appear normal [21]. Yet, in patients with acute pancreatitis, abnormalities on ultrasonography may be detected in 33%-90% of cases. Suggested abnormal findings include enlargement of the pancreas with changes in the echogenicity, dilatation of the pancreatic duct, and the presence of fluid collection (Fig. 10) [22]. Furthermore, ultrasonography is invaluable in investigating potential predisposing factors of acute pancreatitis, such as gallstones or fatty liver. Fatty liver is an indicator of hypertriglyceridemia, which is one of the major causes of acute pancreatitis. It also aids in detecting complications such as vascular complications or abscesses (Fig. 11). Vascular complications include thrombosis and pseudoaneurysms, in which the splenic vein and artery are commonly involved, respectively. Peripancreatic fluid collections may appear purely anechoic or may contain echogenic debris, which may be the result of hemorrhage, necrosis, or infection (Figs. 10, 11) [18].
- Abdominal Aortic Aneurysm
- Abdominal Aortic Aneurysm
Most abdominal aortic aneurysms (AAAs) are infrarenal [23]. In adults, an aortic diameter of more than 3.0 cm is generally considered an aneurysm [24]. Risk factors include male sex, smoking, hypertension, diabetes mellitus, coronary artery disease, and a family history of AAA [25]. While AAA often remains asymptomatic, a rupture transforms it into a medical emergency, with mortality rates ranging from 50% to 90% [26]. The majority of the AAAs are fusiform, and a mural thrombus may be seen (Fig. 12). Saccular aneurysms can hint at a mycotic aneurysm (Fig. 13). Ultrasonography is the primary tool for AAA screening due to its commendable sensitivity (95%) and unparalleled specificity (100%) [27]. Color Doppler ultrasonography may show the turbulent flow in the aortic aneurysm (Fig. 14). While CT angiography remains the gold standard for detecting ruptured AAAs (Fig. 15), point-of-care ultrasonography (PoCUS) can also provide excellent sensitivity (97.8%) and specificity (97.0%) in patients suspected of having ruptured AAAs (Fig. 13) [28]. Although PoCUS might not directly visualize the rupture, indirect signs such as retroperitoneal hematoma may offer crucial information that can hasten diagnostic processes and facilitate timely medical interventions for symptomatic individuals [28].
- Acute Colonic Diverticulitis
- Acute Colonic Diverticulitis
The ascending colon and descending colon are anchored to the retroperitoneum. They run a vertical course in the abdomen, positioned anteriorly to the iliopsoas muscles. On ultrasonography, a haustration pattern can be observed in the colon. The ascending colon frequently contains fecal materials and gas. Conversely, the descending colon typically remains contracted, making its bowel wall more discernible via ultrasonography. Generally, the normal diameter of the colon is less than 5 cm, with wall thickness between 0.5 and 4 mm [29].On ultrasonography, five layers can be observed in the bowel wall due to the echoic interactions of histological layers and interfaces. The first hyperechoic layer is the interface between the bowel lumen and the mucosa. The mucosa is hypoechoic and constitutes the second layer. The third layer is the submucosa, and it is hyperechoic. The fourth layer (hypoechoic) corresponds to the muscularis propria. The fifth layer is hyperechoic and represents the interface between the muscularis and the serosa [30].Colonic diverticula are a common finding, especially in the elderly, with an estimated prevalence rate of 65% in people older than 80 years [31]. The clinical presentation of acute diverticulitis includes lower abdominal pain (often left-sided), abdominal tenderness and fever. Elevated serum inflammatory markers often accompany these symptoms [32]. Imaging is usually required to confirm the diagnosis. Both ultrasonography and CT can be used for diagnosis, with comparable sensitivity and specificity [33]. On ultrasonography, the colonic diverticula can be found as outpouchings from the colonic wall that contain echogenic foci. A fecalith within these outpouchings is characterized by an acoustic shadow [34]. The hallmark ultrasonographic features of acute diverticulitis include an inflamed diverticulum paired with localized, segmental bowel wall thickening and nearby pericolic stranding. These signs often align with the region of most intense pain, which can be further pinpointed by applying gentle pressure with the ultrasound probe [31,34]. The appearance of an inflamed diverticulum can vary; most commonly, it features a hyperechoic surrounded by a hypoechoic rim (41%) or is entirely hypoechoic (37%) [35]. While diverticulitis may evolve and lead to retroperitoneal abscess formation (Fig. 16). Colon cancer remains a crucial consideration in the differential diagnosis, and differentiating between the two conditions can be challenging. Typically, a colonoscopy is recommended once the acute inflammatory phase has subsided.
- Retroperitoneal Appendicitis
- Retroperitoneal Appendicitis
Appendicitis is a commonly encountered etiology of acute abdomen. The anatomical position of the appendix is predominantly intraperitoneal; however, the literature documents its retroperitoneal localization in 30%-65% of instances [36]. Several ultrasound signs suggesting acute appendicitis had been proposed, such as the maximum diameter of the appendix larger than 6 mm, an incompressible appendix, the presence of a large appendicolith, and increased appendiceal wall vascularity on Doppler imaging [37- 40]. Peri-appendiceal fat strandings result in an increasing periappendicular echogenicity. Features indicating perforation consist of the loss of the echogenic submucosal layer and loculated periappendicular abscess [41].
- Ischemic Colitis
- Ischemic Colitis
Ischemic colitis is the most frequent form of bowel ischemia. Long-segmental circumferential bowel wall thickening and loss of mural stratification can be observed on ultrasonography [42]. Both ultrasonography and CT reveal comparable accuracy in diagnosing ischemic colitis, but CT holds a distinct advantage in detecting pneumatosis [43]. The sensitivity of ultrasonography in detecting ischemic colitis is high (95%) [44]. However, it is pivotal to recognize that sonographic findings in ischemic colitis can often mirror those observed in other gastrointestinal disorders. The absence or faint visibility of flow signals on Doppler can provide crucial insights, strengthening diagnostic confidence in suspected cases of ischemic colitis [42,45].
- Retroperitoneal Abscess and Hematoma
- Retroperitoneal Abscess and Hematoma
The retroperitoneal space, characterized by its relatively loose structure and limited blood circulation, is susceptible to infections, often leading to abscess formation [46]. The causes of these infections in the retroperitoneal region vary widely and can arise from gastrointestinal tract perforations (Fig. 16), urinary tract infections (Figs. 8, 9, 17), acute pancreatitis (Fig. 11), and others (Fig. 18). Notably, spinal osteomyelitis can result in psoas muscle abscesses [47]. The challenge with retroperitoneal abscesses lies in their clinical manifestations; they are diverse and often lack distinctive signs, complicating the diagnosis. Ultrasonography can detect local soft tissue edema and abscess formation [48]. In acute stages, the abscess may appear as a round or oval lesion with thick walls and heterogeneous internal echogenicity due to cellular debris and proteinaceous fluid [49]. Moreover, ultrasonography can be a pivotal guide for inserting drainage catheters [47].In contrast, retroperitoneal hematomas (Figs. 19, 20) may be the result of trauma, medical procedures, or can emerge spontaneously, particularly in patients on antiplatelet or anticoagulant therapies [50,51]. In addition, gynecologic or obstetric conditions, including hemorrhagic cysts, ectopic pregnancies, or vaginal deliveries, can give rise to these hematomas [52,53]. A study by Yeoh et al. [54] highlighted that patients might not display overt clinical signs or symptoms until significant blood loss, underscoring the importance of maintaining a high level of clinical suspicion for retroperitoneal hematomas. An early and accurate diagnosis is imperative to mitigate severe or potentially fatal outcomes [55]. The ultrasonographic appearance of hematomas can change over different stages, making it tricky at times to differentiate them from fluid collections like abscesses solely based on ultrasonography. Hence, understanding the clinical context is vital for timely and accurate diagnosis [56].
- Advantages and Limitations of Ultrasonography
- Advantages and Limitations of Ultrasonography
Ultrasonography is an excellent diagnostic modality due to its portability. This makes it particularly useful for critically ill patients who may not be safely transported to suites for CT. Additionally, ultrasonography has strength of non-invasiveness, lower cost, multiplanar capability, and non-ionizing radiation. However, ultrasonography is a user-dependent imaging technique. A comprehensive examination of the retroperitoneum can sometimes be very challenging, even for experienced physicians, due to the hindrance of bowel gas. Due to the absence of ionizing radiation, ultrasonography is also an optimal tool for evaluating pediatric patients and pregnant women, who are more susceptible to radiation exposure. In obese patients, the effectiveness of ultrasonography diminishes due to reduced penetration of the ultrasound waves (Fig. 15). In contrast, CT is another prevalent imaging tool for assessing acute retroperitoneal conditions. When juxtaposed with ultrasonography, CT exhibits higher accuracy, a comprehensive view, reduced operator bias, a more precise depiction of soft tissue or fascial changes (Figs. 15, 17), higher sensitivity in delineating renal infarcts or parenchymal changes (Fig. 21), and higher specificity in delineating abnormal gas collection (Figs. 15, 18) or urolithiasis.
- Conclusion
- Conclusion
A variety of underlying causes can lead to acute retroperitoneal conditions, and ultrasonography is an important tool for evaluating patients due to its wide availability and accessibility. Understanding the anatomy of the retroperitoneum and the imaging characteristics of acute retroperitoneum is the key to an early and accurate diagnosis. When coupled with relevant clinical data and laboratory results, physicians can better render precise diagnoses and tailor effective treatments for their patients.
- NOTES
- NOTES
-
Author Contributions Conceptualization: Lin G, Wang LJ, Wan YL. Data acquisition: Liu HH, Wan YL. Data analysis or interpretation: Liu HH, Lin Y. Drafting of the manuscript: Liu HH, Lin Y. Critical revision of the manuscript: Lin G, Wang LJ, Wan YL. Approval of the final version of the manuscript: all authors.
No potential conflict of interest relevant to this article was reported.
Fig. 1.
A case of acute left flank pain due to left upper ureteral stone.
A. Coronal sonography of the left kidney shows left hydronephrosis (asterisk) due to echogenic stones identified in the left upper ureter (arrow) in (B).

Fig. 2.
A subject with patent ureters.
Transverse ultrasonography of the bladder using advanced dynamic flow shows an urine jet as a distinct stream of urine entering the bladder.
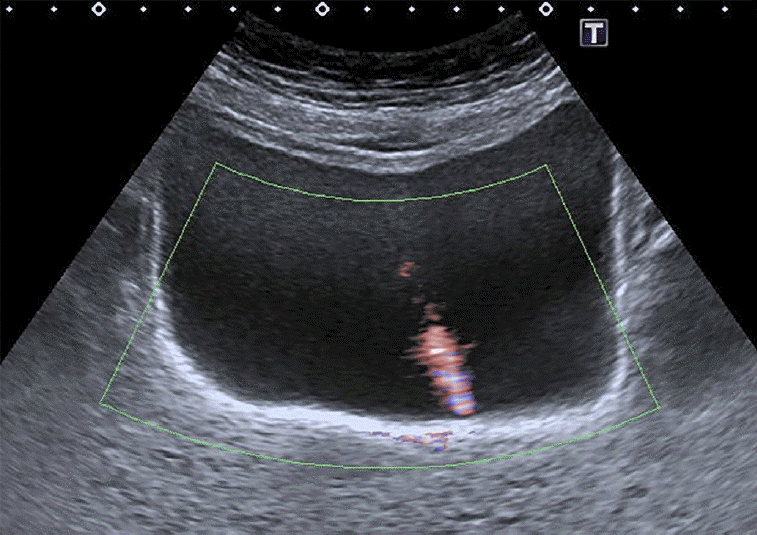
Fig. 3.
A case of acute pyelonephritis.
Ultrasonography of the kidney shows swelling of the left kidney with hypoechoic foci (arrows) and loss of cortico-medullary differentiation, consistent with acute pyelonephritis.
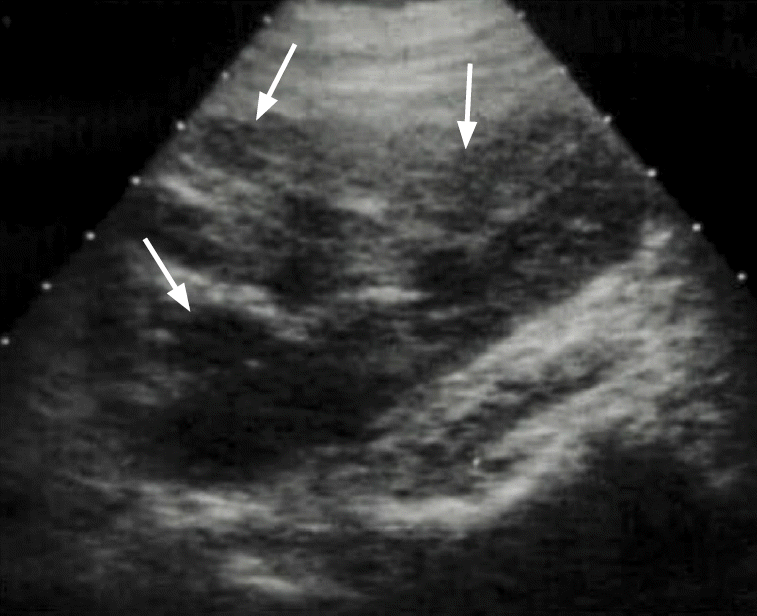
Fig. 4.
A patient presented with fever, flank pain and pyuria.
Ultrasonography shows swelling and hypoechoic lesion (arrow) in the upper pole of the kidney, consistent with acute pyelonephritis.
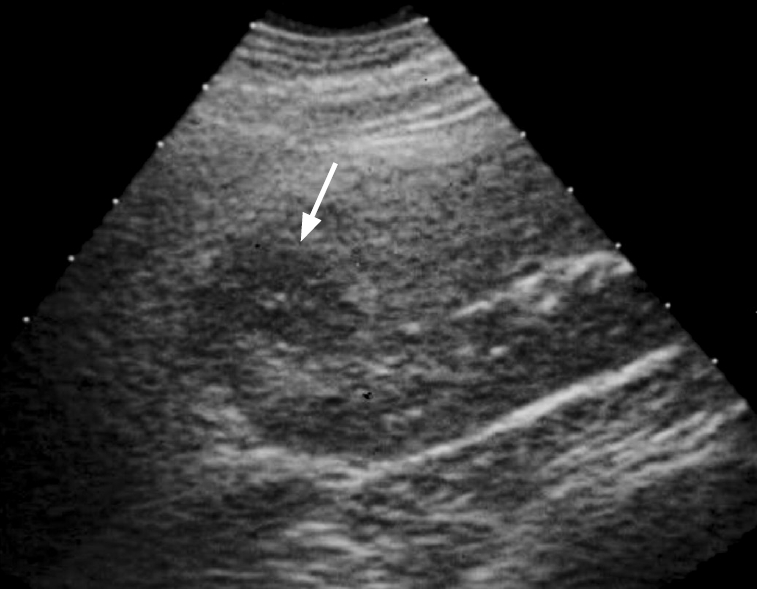
Fig. 5.
A case of renal abscess.
A. Ultrasonography of the right kidney shows a well-defined hypoechoic mass (white arrows) due to renal abscess. B. Contrast-enhanced computed tomography of the right kidney shows a well-defined hypodense mass with an enhanced wall (black arrows) due to renal abscess.
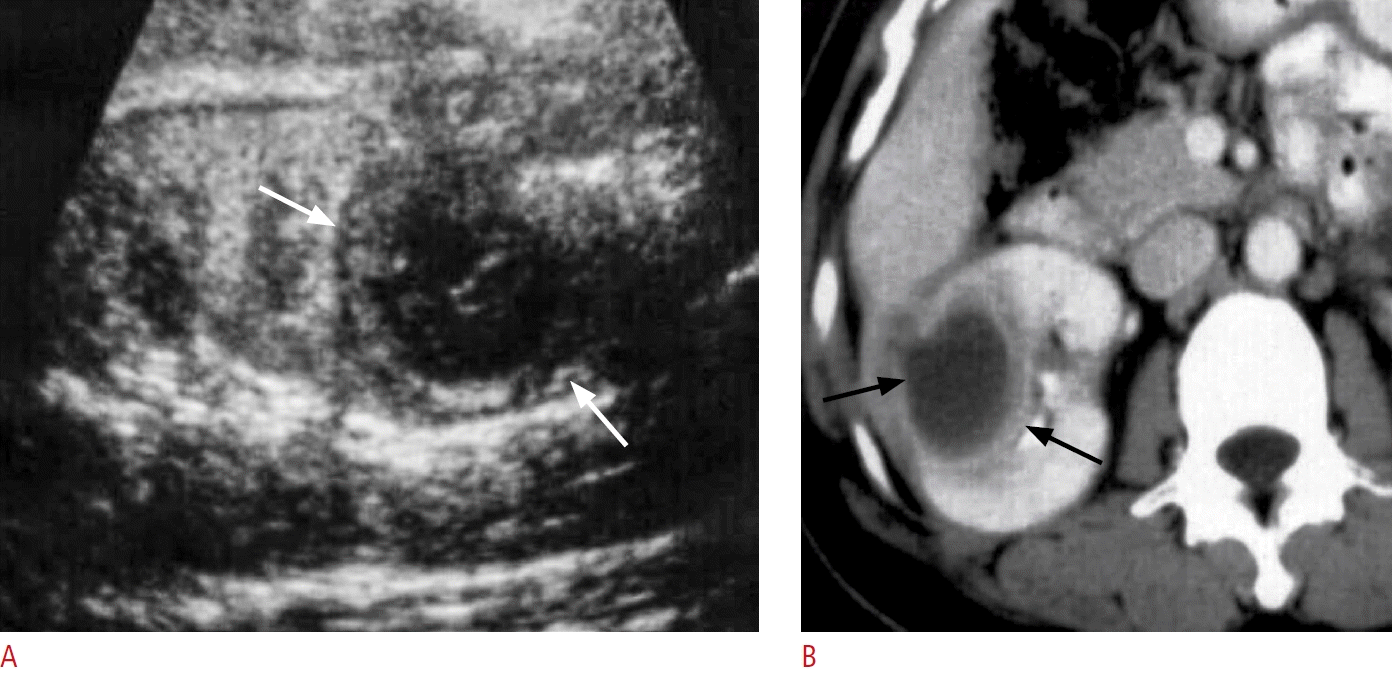
Fig. 6.
A case of pyonephrosis and pyoureter.
A. Ultrasonography of the right kidney shows dilated right upper collecting system with fluid-fluid layering (arrow) suggesting pyocalyx. B. Sagittal ultrasonography of the pelvis in another patient shows ureterocele and pyouretrer with internal echoes due to suppurative echogenic debris (arrows) in the obstructed urotract suggesting pyonephrosis and pyoureter.
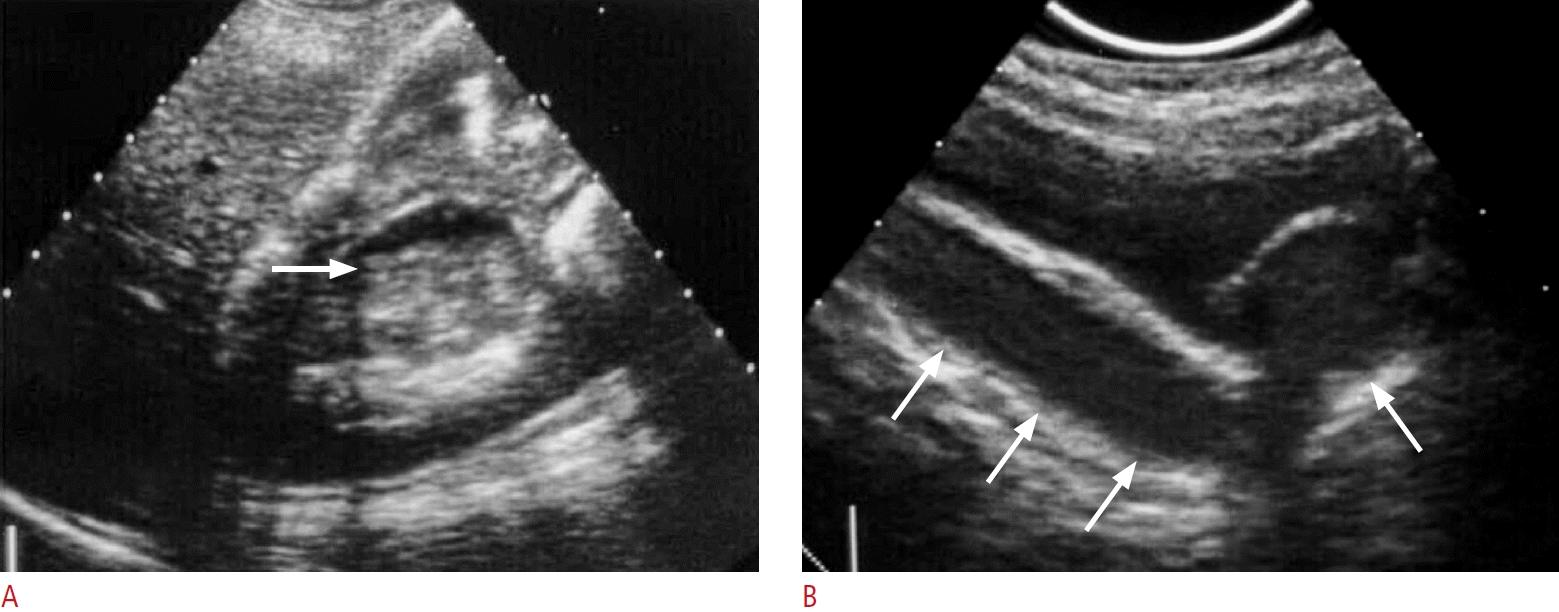
Fig. 7.
A case of type I emphysematous pyelonephritis.
A. Ultrasonography of the left kidney shows complete obscuration of the left kidney by the curvilinear high echogenicity (arrows) due to gas. B. Computed tomography shows complete destruction of the left renal parenchymal with gas content (arrows).
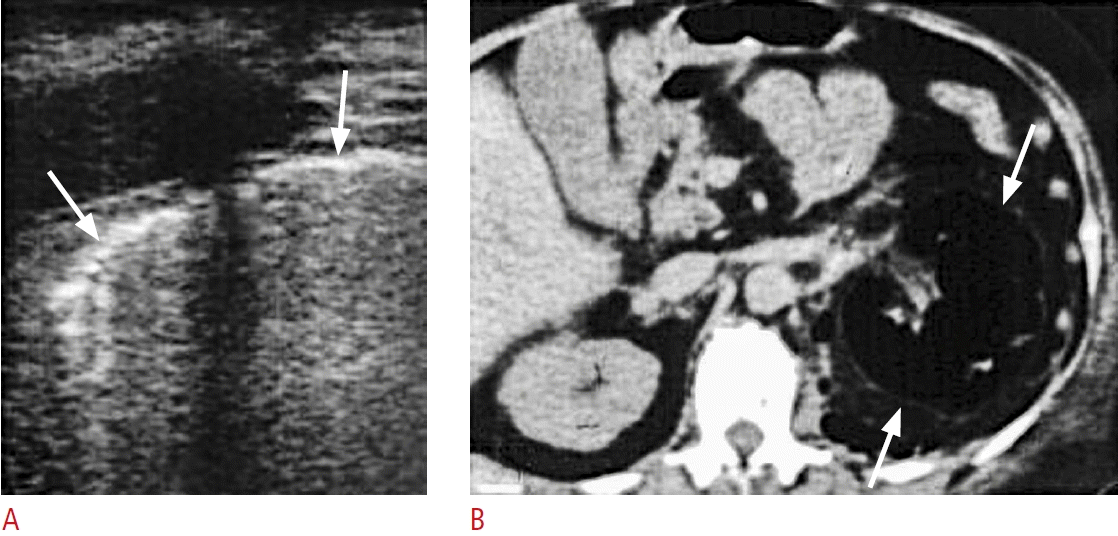
Fig. 8.
A case of type II emphysematous pyelonephritis.
A. Radiography of the abdomen shows loculated and bubbly gas (arrows) in the right renal area. B. Computed tomography with a modified lung window level shows gas in collecting system (white arrows) as well as gas and fluid content in the right kidney (black arrowhead) and perirenal space (black arrow).
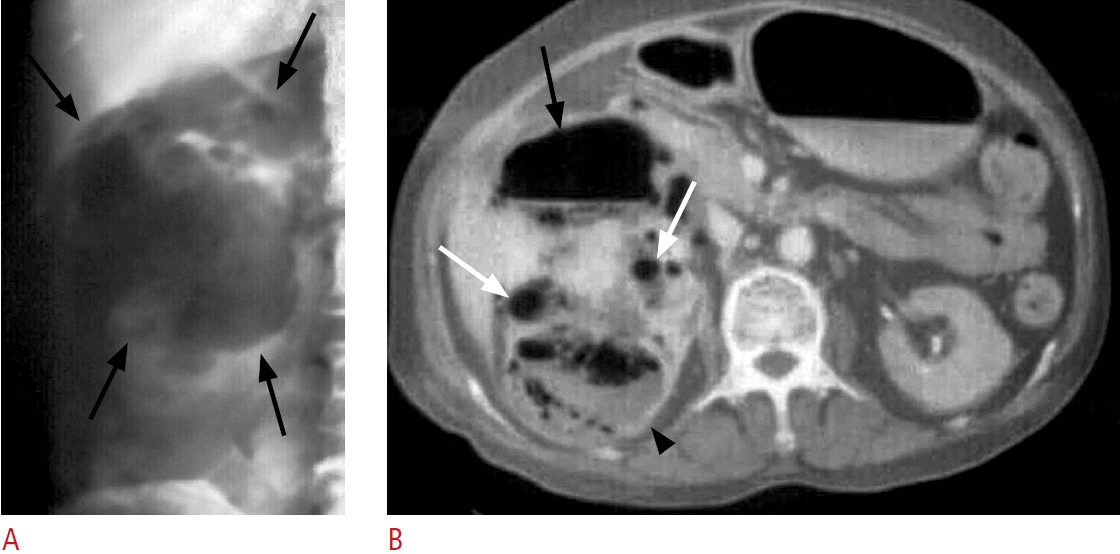
Fig. 9.
A case of type II emphysematous pyelonephritis.
A. Ultrasonography shows echogenic foci in the left renal calyx (black arrow) and perirenal space (white arrows) due to gas content. B. Post-enhanced computed tomography shows gas in the collecting systems (black arrows) and perirenal space (white arrows) with subtle fluid content.

Fig. 10.
A case of acute pancreatitis.
A. Coronal ultrasonography of left upper abdomen reveals anechoic fluid collection in the left anterior pararenal space (arrows). B. Contrastenhanced computed tomography of the same patient shows fluid collection (arrow) in the left anterior pararenal space due to acute pancreatitis.
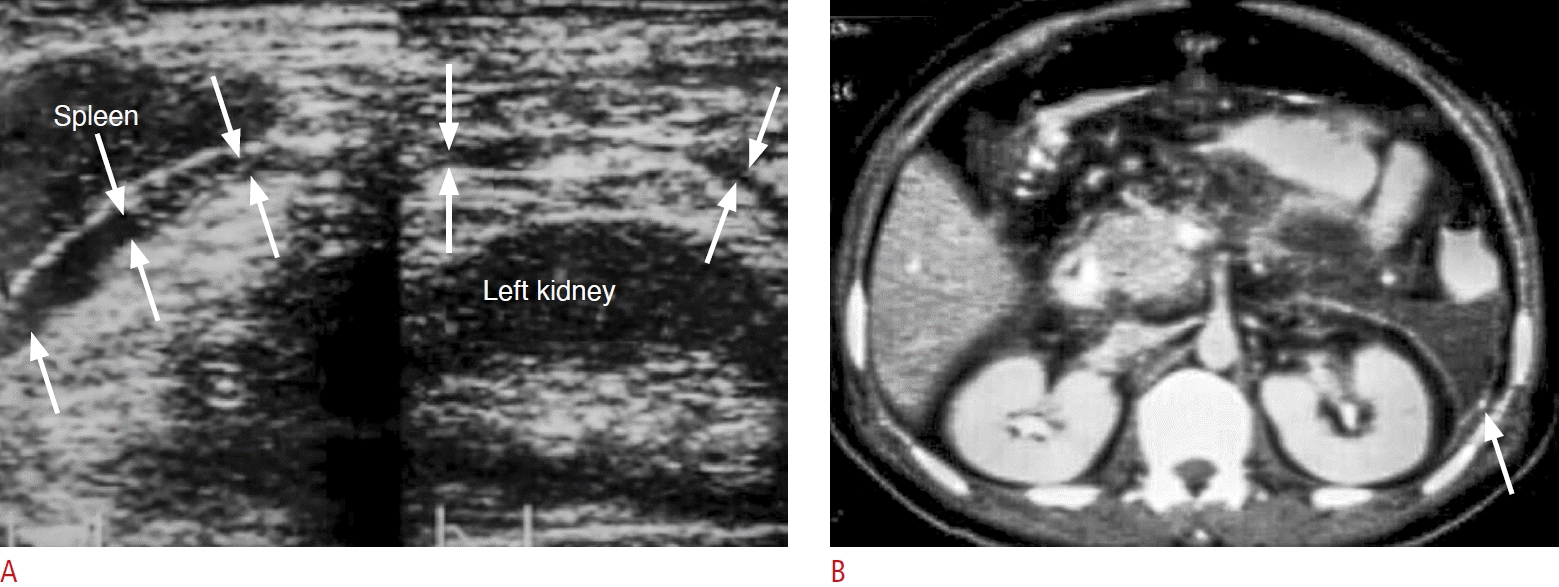
Fig. 11.
A case of acute pancreatitis complicated with retroperitoneal abscess in the left anterior pararenal space.
A. Coronal sonography of the left retroperitoneum shows hypoechoic abscess in the left retroperitoneum (arrows) lateral to the psoas muscle. B. Contrast-enhanced computed tomography confirms abscesses in in the left anterior pararenal space (arrows).

Fig. 12.
A case of mycotic aneurysm of abdominal aorta in a diabetic patient with periumbilical pain.
A. Right coronal ultrasonography of the retroperitoneum shows a hypoechoic lesion (arrow) adjacent to the aortoiliac arteries. B. Axial image of contrast-enhanced computed tomography of the same patient reveals a mycotic aneurysm (arrow) proved by surgery and bacterial culture.
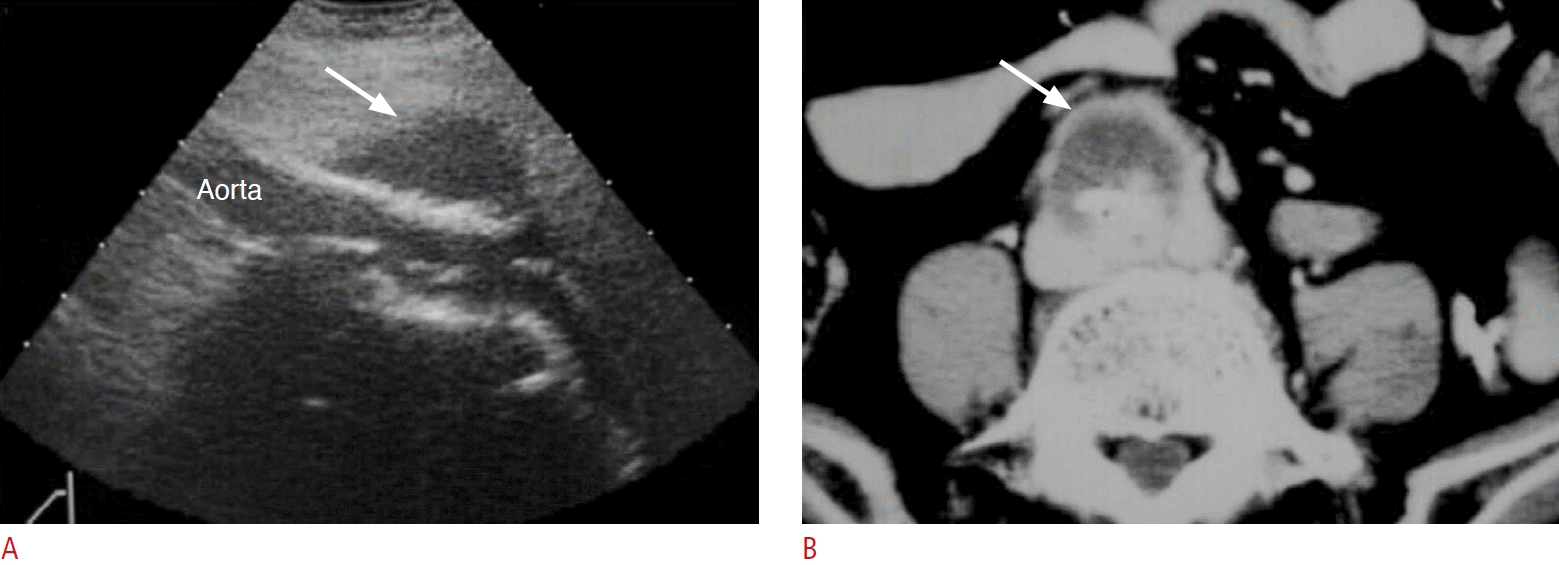
Fig. 13.
Mycotic aneurysm of the infrarenal aorta complicated with rupture.
A. Left coronal sonography of the retroperitoneum shows a round hypoechoic mass due to abdominal aortic aneurysm with wall interruption (arrow). The aneurysm is associated with surrounding soft tissue or hematoma. B. Axial view of contrast-enhanced computed tomography shows rupture of the mycotic aortic aneurysm with wall interruption (arrowhead) and retroperitoneal hematoma (arrows).

Fig. 14.
A case of pulsating abdominal mass.
Color Doppler ultrasonography shows a Yin-Yang sign in the aorta suggestive of abdominal aortic aneurysm.
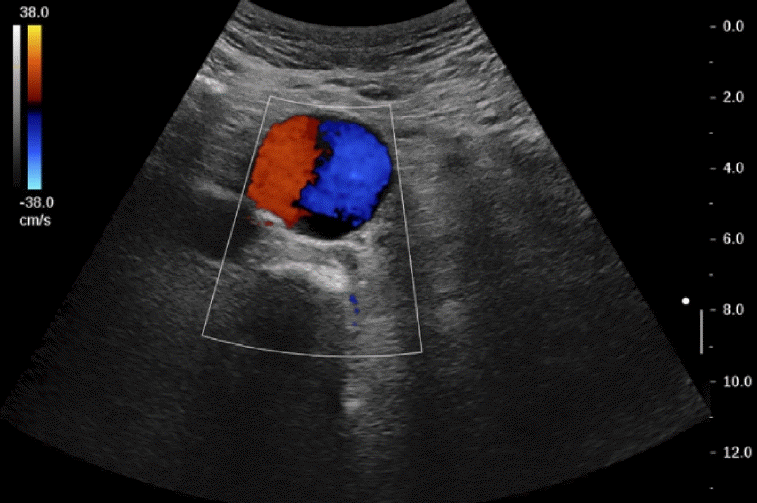
Fig. 15.
Two different obese diabetics with mycotic aortic aneurysms which could not be delineated by ultrasonography.
A. Contrast-enhanced computed tomography of the abdomen shows indistinct outline of the abdominal aorta with surrounding soft tissue strands (arrow) suggesting either aortic leak or mycotic aortic aneurysm. B. Contrast-enhanced computed tomography shows indistinct outline of the abdominal aorta with gas collections (arrows) indicating mycotic aortic aneurysm. The gas could not be well shown by ultrasound.
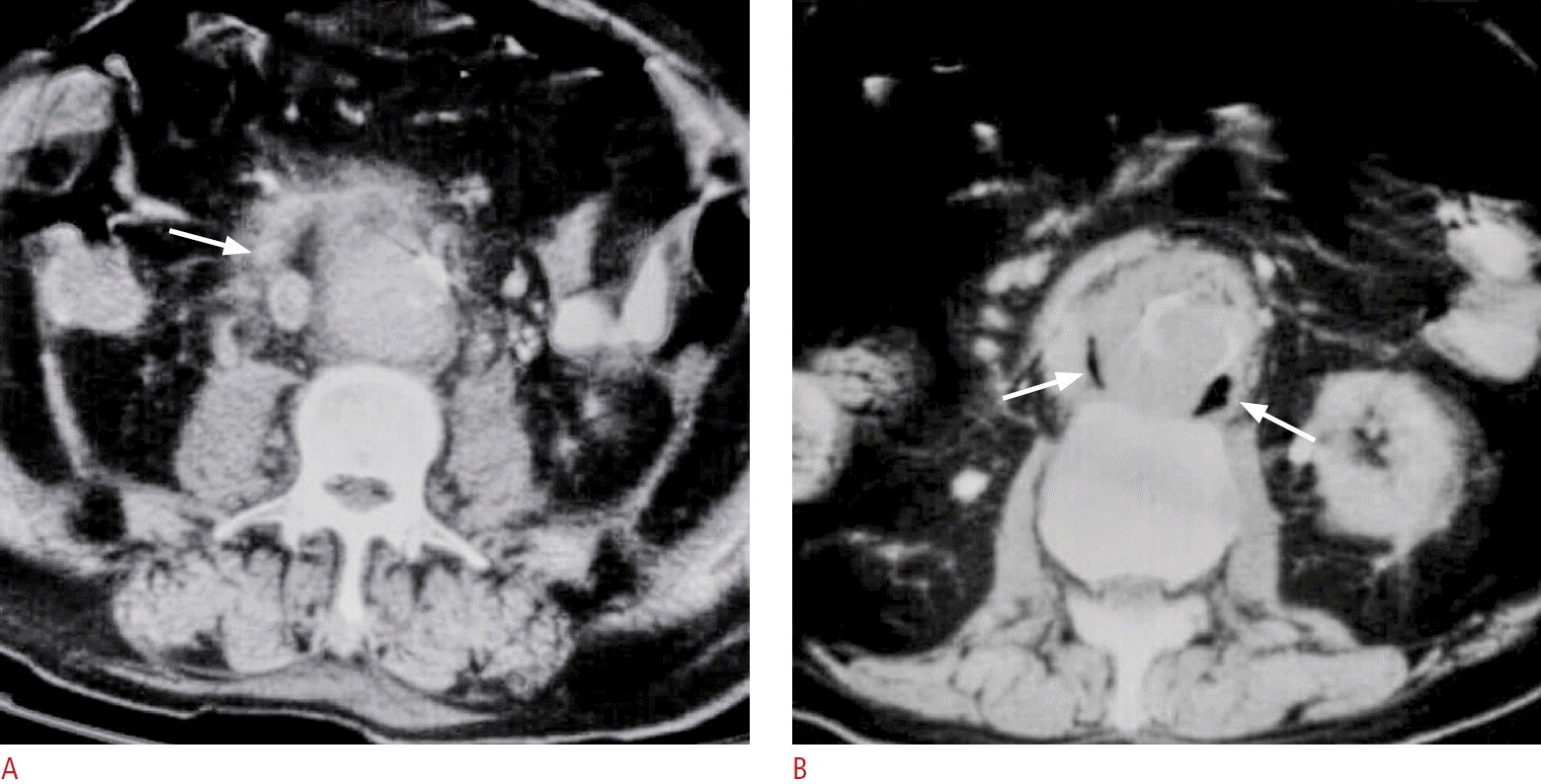
Fig. 16.
A case of retroperitoneal abscess due to complicated diverticulitis of the descending colon.
A. Coronal ultrasonography of the left abdomen shows hypoechoic masses or fluid collections with internal echoes in the left retroperitoneum involving the left posterior pararenal space, psoas muscle (black arrows) and abdominal wall (white arrows). B. Contrastenhanced computed tomography shows abscesses with enhanced wall involving the left posterior pararenal space, left psoas muscle (black arrows) and left abdominal wall (white arrows).

Fig. 17.
A diabetic patient with gas-containing perirenal abscess presenting with right flank pain, fever and chills.
A. The lesion was not diagnosed by ultrasonography initially. B. Axial contrast-enhanced computed tomography performed one day after ultrasound shows right perirenal abscess with thick enhanced fascia (white arrows), gas contents (black arrows) and fluid collections that were not well detected by initial ultrasonography (A). P, abscess in the perirenal space; RK, right kidney.
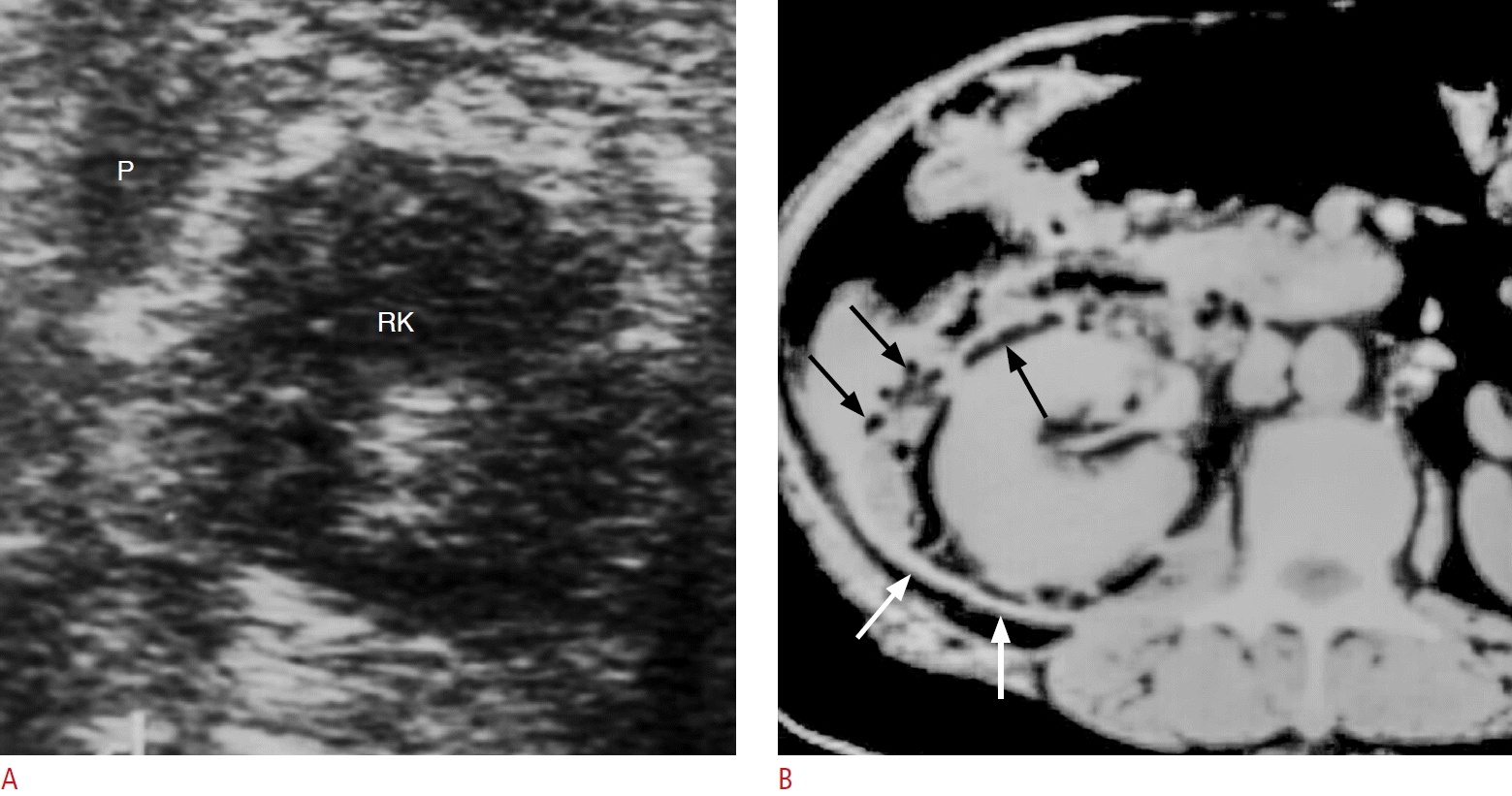
Fig. 18.
A case of retroperitoneal abscess in the psoas muscle.
A. Right coronal ultrasonography shows multiple echogenic foci (arrows) in the right psoas muscle due to gas. B. Sequential axial view of contrast-enhanced computed tomography reveals a gas-containing abscess in the right psoas muscle (arrows).

Fig. 19.
A case of post-traumatic peri-renal hematoma.
Ultrasonography of the left kidney reveals post-traumatic laceration of the left renal parenchyma (black arrows) with hematoma at the perirenal area (white arrows).
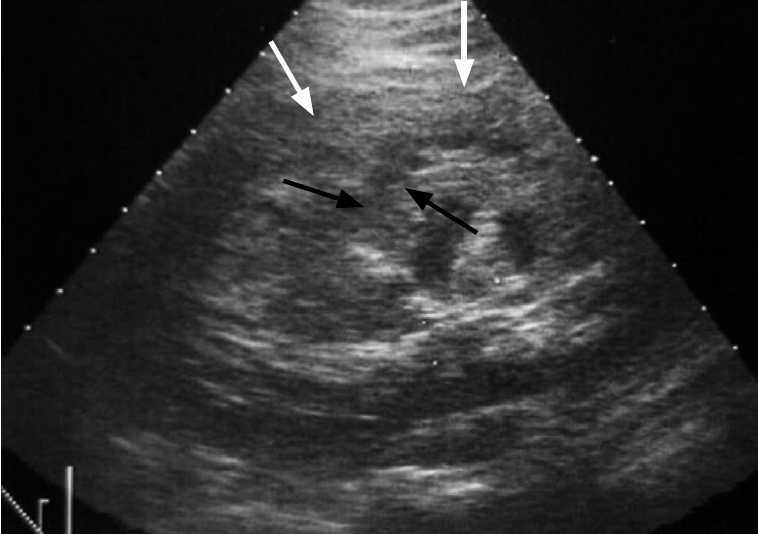
Fig. 20.
A case of left renal hamartoma with spontaneous rupture presenting as acute left flank pain.
Ultrasonography of the left renal area shows a hyperechoic mass due to hamartoma (white arrow) with heterogenous lesion surrounding the left kidney due to hematoma (black arrows).
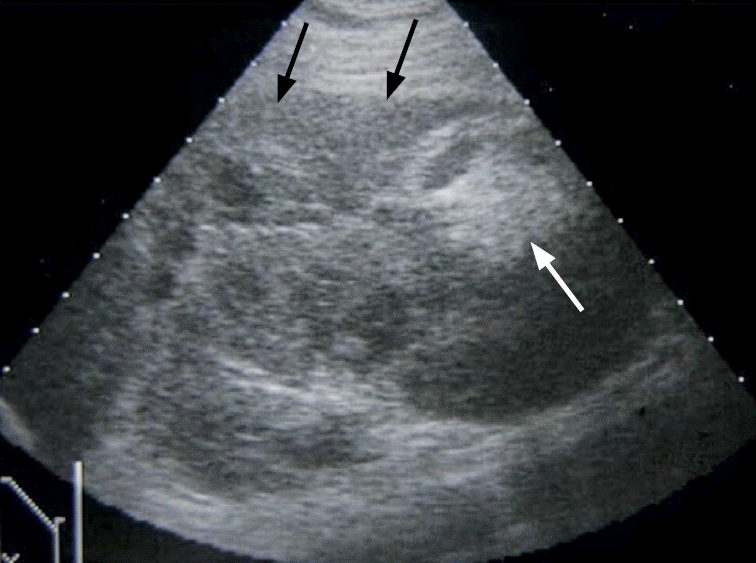
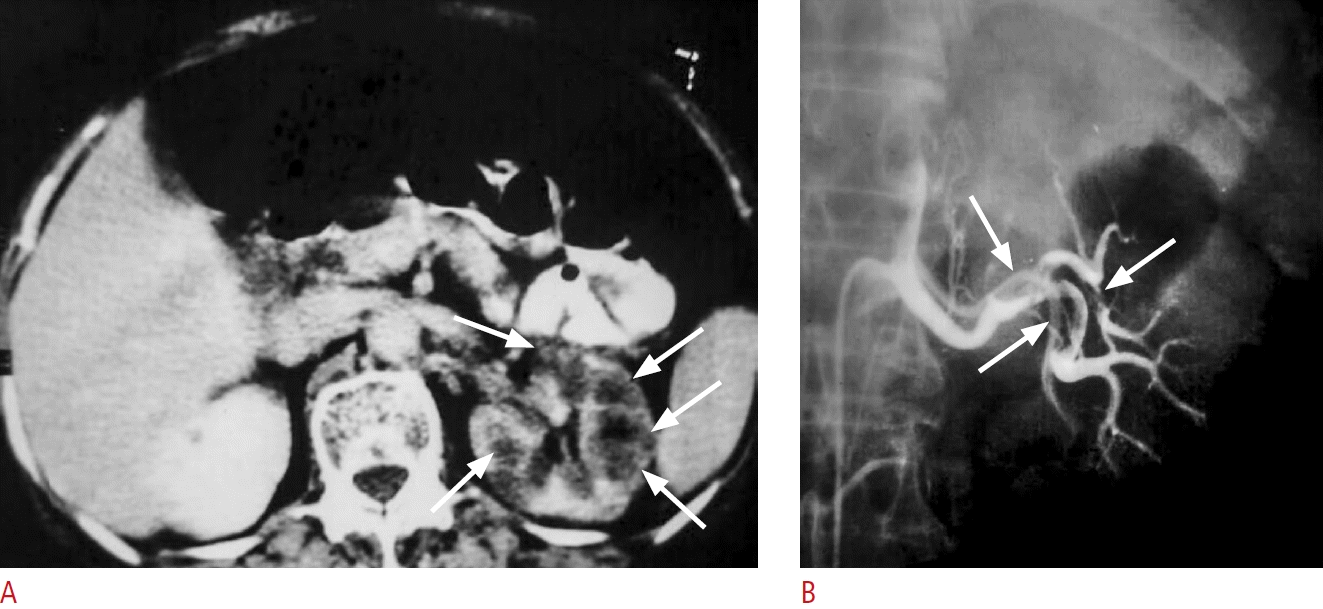
Fig. 21.
A case of acute left flank pain with hematuria due to emboli from the left cardiac atrium.
A. Contrast-enhanced computed tomography shows hypodense areas (arrows) in the left renal parenchyma which is consistent with renal infarct. The findings were not detected by ultrasound (not shown). B. Transcatheter left renal angiography displays filling defects (arrows) in the left main and segmental renal arteries.
- REFERENCES
- REFERENCES
References
1. Moe OW. Kidney stones: pathophysiology and medical management. Lancet 2006;367:333–344.
[Article] [PubMed]2. Corbo J, Wang J. Kidney and ureteral stones. Emerg Med Clin North Am 2019;37:637–648.
[Article] [PubMed]3. Tamm EP, Silverman PM, Shuman WP. Evaluation of the patient with flank pain and possible ureteral calculus. Radiology 2003;228:319–329.
[Article] [PubMed]4. Ahmed F, Askarpour MR, Eslahi A, Nikbakht HA, Jafari SH, Hassanpour A, et al. The role of ultrasonography in detecting urinary tract calculi compared to CT scan. Res Rep Urol 2018;10:199–203.
[Article] [PubMed] [PMC]5. Aghaways I, Ibrahim R, Bapir R, Salih RQ, Salih KM, Abdulla BA. The role of inflammatory serum markers and ureteral wall thickness on spontaneous passage of ureteral stone < 10 mm: a prospective cohort study. Ann Med Surg (Lond) 2022;80:104198.
[Article] [PubMed] [PMC]6. Smith-Bindman R, Aubin C, Bailitz J, Bengiamin RN, Camargo CA Jr, Corbo J, et al. Ultrasonography versus computed tomography for suspected nephrolithiasis. N Engl J Med 2014;371:1100–1110.
[Article] [PubMed]7. Turk C, Petrik A, Sarica K, Seitz C, Skolarikos A, Straub M, et al. EAU guidelines on diagnosis and conservative management of urolithiasis. Eur Urol 2016;69:468–474.
[Article] [PubMed]8. Expert Panel on Urological Imaging; Smith AD, Nikolaidis P, Khatri G, Chong ST, De Leon AD, et al. ACR Appropriateness Criteria(R) acute pyelonephritis: 2022 update. J Am Coll Radiol 2022;19(11S):S224–S239.
[PubMed]9. Zulfiqar M, Ubilla CV, Nicola R, Menias CO. Imaging of renal infections and inflammatory disease. Radiol Clin North Am 2020;58:909–923.
[Article] [PubMed]10. Farmer KD, Gellett LR, Dubbins PA. The sonographic appearance of acute focal pyelonephritis 8 years experience. Clin Radiol 2002;57:483–487.
[Article] [PubMed]11. Kawashima A, LeRoy AJ. Radiologic evaluation of patients with renal infections. Infect Dis Clin North Am 2003;17:433–456.
[Article] [PubMed]12. Rai RS, Karan SC, Kayastha A. Renal and perinephric abscesses revisited. Med J Armed Forces India 2007;63:223–225.
[Article] [PubMed] [PMC]13. Yen DH, Hu SC, Tsai J, Kao WF, Chern CH, Wang LM, et al. Renal abscess: early diagnosis and treatment. Am J Emerg Med 1999;17:192–197.
[Article] [PubMed]14. Liu XQ, Wang CC, Liu YB, Liu K. Renal and perinephric abscesses in West China Hospital: 10-year retrospective-descriptive study. World J Nephrol 2016;5:108–114.
[Article] [PubMed] [PMC]15. Hill GS. Renal infection. In: Hill GS, ed. Uropathology. New York: Churchill Livingstone, 1989;33.16. Wan YL, Lee TY, Bullard MJ, Tsai CC. Acute gas-producing bacterial renal infection: correlation between imaging findings and clinical outcome. Radiology 1996;198:433–438.
[Article] [PubMed]17. Wan YL, Lo SK, Bullard MJ, Chang PL, Lee TY. Predictors of outcome in emphysematous pyelonephritis. J Urol 1998;159:369–373.
[Article] [PubMed]18. Rumack CM, Levine D. Diagnostic ultrasound. 5th ed. Philadelphia: Elsevier Health Sciences, 2017.19. Glaser J, Stienecker K. Pancreas and aging: a study using ultrasonography. Gerontology 2000;46:93–96.
[Article] [PubMed]20. Expert Panel on Gastrointestinal Imaging, Porter KK, Zaheer A, Kamel IR, Horowitz JM, Arif-Tiwari H, et al. ACR Appropriateness Criteria(R) acute pancreatitis. J Am Coll Radiol 2019;16(11S):S316–S330.
[PubMed]21. Hertzberg BS, Middleton WD. Ultrasound: the requisites. 3rd ed. New York: Elsevier Health Sciences, 2015.22. Balthazar EJ. Acute pancreatitis: assessment of severity with clinical and CT evaluation. Radiology 2002;223:603–613.
[Article] [PubMed]23. Jongkind V, Yeung KK, Akkersdijk GJ, Heidsieck D, Reitsma JB, Tangelder GJ, et al. Juxtarenal aortic aneurysm repair. J Vasc Surg 2010;52:760–767.
[Article] [PubMed]24. Berland LL, Silverman SG, Gore RM, Mayo-Smith WW, Megibow AJ, Yee J, et al. Managing incidental findings on abdominal CT: white paper of the ACR Incidental Findings Committee. J Am Coll Radiol 2010;7:754–773.
[PubMed]25. Altobelli E, Rapacchietta L, Profeta VF, Fagnano R. Risk factors for abdominal aortic aneurysm in population-based studies: a systematic review and meta-analysis. Int J Environ Res Public Health 2018;15:2805.
[Article] [PubMed] [PMC]26. Kent KC. Clinical practice: abdominal aortic aneurysms. N Engl J Med 2014;371:2101–2108.
[Article] [PubMed]27. Fleming C, Whitlock EP, Beil TL, Lederle FA. Screening for abdominal aortic aneurysm: a best-evidence systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2005;142:203–211.
[Article] [PubMed]28. Fernando SM, Tran A, Cheng W, Rochwerg B, Strauss SA, Mutter E, et al. Accuracy of presenting symptoms, physical examination, and imaging for diagnosis of ruptured abdominal aortic aneurysm: systematic review and meta-analysis. Acad Emerg Med 2022;29:486–496.
[Article] [PubMed]29. Hollerweger A. Colonic diseases: the value of US examination. Eur J Radiol 2007;64:239–249.
[Article] [PubMed]30. Nylund K, Maconi G, Hollerweger A, Ripolles T, Pallotta N, Higginson A, et al. EFSUMB recommendations and guidelines for gastrointestinal ultrasound. Ultraschall Med 2017;38:e1–e15.
[PubMed]31. Maconi G, Carmagnola S, Guzowski T. Intestinal ultrasonography in the diagnosis and management of colonic diverticular disease. J Clin Gastroenterol 2016;50 Suppl 1:S20–S22.
[Article] [PubMed]32. Cuomo R, Barbara G, Pace F, Annese V, Bassotti G, Binda GA, et al. Italian consensus conference for colonic diverticulosis and diverticular disease. United European Gastroenterol J 2014;2:413–442.
[Article] [PubMed] [PMC]33. Andeweg CS, Wegdam JA, Groenewoud J, van der Wilt GJ, van Goor H, Bleichrodt RP. Toward an evidence-based step-up approach in diagnosing diverticulitis. Scand J Gastroenterol 2014;49:775–784.
[Article] [PubMed]34. Valentino M, Serra C, Ansaloni L, Mantovani G, Pavlica P, Barozzi L. Sonographic features of acute colonic diverticulitis. J Clin Ultrasound 2009;37:457–463.
[Article] [PubMed]35. Hollerweger A, Macheiner P, Rettenbacher T, Brunner W, Gritzmann N. Colonic diverticulitis: diagnostic value and appearance of inflamed diverticula-sonographic evaluation. Eur Radiol 2001;11:1956–1963.
[Article] [PubMed]36. Nahhas A, Abderahman A, Kharaba A. A snake in the grass: retroperitoneal abscess due to perforated appendicitis-management, approach and recommendations. J Surg Case Rep 2019;2019:rjz163.
[Article] [PubMed] [PMC]37. Rettenbacher T, Hollerweger A, Macheiner P, Rettenbacher L, Tomaselli F, Schneider B, et al. Outer diameter of the vermiform appendix as a sign of acute appendicitis: evaluation at US. Radiology 2001;218:757–762.
[Article] [PubMed]38. Rioux M. Sonographic detection of the normal and abnormal appendix. AJR Am J Roentgenol 1992;158:773–778.
[Article] [PubMed]39. Sivit CJ. Diagnosis of acute appendicitis in children: spectrum of sonographic findings. AJR Am J Roentgenol 1993;161:147–152.
[Article] [PubMed]40. Xu Y, Jeffrey RB, Shin LK, DiMaio MA, Olcott EW. Color Doppler imaging of the appendix: criteria to improve specificity for appendicitis in the borderline-size appendix. J Ultrasound Med 2016;35:2129–2138.
[PubMed]41. Choi J, Kim HJ, Jang SK, Kim HJ, Yeon JW. Useful ultrasound findings of pediatric patients with equivocal results of appendicitis: analysis based on a structured report form. Taehan Yongsang Uihakhoe Chi 2021;82:182–193.
[Article] [PubMed] [PMC]42. Pastor-Juan MD, Ripolles T, Marti-Bonmati L, Martinez MJ, Simo L, Gomez D, et al. Predictors of severity in ischemic colitis: usefulness of early ultrasonography. Eur J Radiol 2017;96:21–26.
[Article] [PubMed]43. Wiesner W, Khurana B, Ji H, Ros PR. CT of acute bowel ischemia. Radiology 2003;226:635–650.
[Article] [PubMed]44. Ripolles T, Simo L, Martinez-Perez MJ, Pastor MR, Igual A, Lopez A. Sonographic findings in ischemic colitis in 58 patients. AJR Am J Roentgenol 2005;184:777–785.
[Article] [PubMed]45. Teefey SA, Roarke MC, Brink JA, Middleton WD, Balfe DM, Thyssen EP, et al. Bowel wall thickening: differentiation of inflammation from ischemia with color Doppler and duplex US. Radiology 1996;198:547–551.
[Article] [PubMed]46. Coffin A, Boulay-Coletta I, Sebbag-Sfez D, Zins M. Radioanatomy of the retroperitoneal space. Diagn Interv Imaging 2015;96:171–186.
[Article] [PubMed]47. Li Z, Tang Y, Wang P, Ren J. Diagnosis and treatment of retroperitoneal infection. Surg Infect (Larchmt) 2021;22:477–484.
[Article] [PubMed]48. Nurnberg D, Mauch M, Spengler J, Holle A, Pannwitz H, Seitz K. Sonographical diagnosis of pneumoretroperitoneum as a result of retroperitoneal perforation. Ultraschall Med 2007;28:612–621.
[PubMed]49. Heller MT, Haarer KA, Thomas E, Thaete F. Acute conditions affecting the perinephric space: imaging anatomy, pathways of disease spread, and differential diagnosis. Emerg Radiol 2012;19:245–254.
[Article] [PubMed]50. Hosseini M, Hosseinzadeh A, Raufian K, Hedjazi A. Nontraumatic retroperitoneal hematoma after Warfarin administration: fatal case report and review of the literature. Am J Forensic Med Pathol 2015;36:245–248.
[PubMed]51. Dubovsky M, Hajska M, Panyko A, Vician M. Severe Retroperitoneal hemorrhage in a COVID-19 patient on a therapeutic dose of low molecular weight heparin: a case report. Cureus 2022;14:e26275.
[PubMed] [PMC]52. De Blasis I, Vinci V, Sergi ME, Capozza F, Saldari M, Moro F, et al. Early and late onset complications of gynaecologic surgery: a multimodality imaging approach. Facts Views Vis Obgyn 2017;9:5–14.
[PubMed] [PMC]53. Maroyi R, Ngeleza N, Kalunga K, Buhendwa C, Shahid U, Boij R, et al. Large retroperitoneal hematoma following vaginal delivery: a case report. J Med Case Rep 2021;15:290.
[Article] [PubMed] [PMC]54. Yeoh WC, Lee KT, Zainul NH, Syed Alwi SB, Low LL. Spontaneous retroperitoneal hematoma: a rare bleeding occurrence in COVID-19. Oxf Med Case Reports 2021;2021:omab081.
[Article] [PubMed] [PMC]
