AbstractPurposeThis study assessed the outcomes of ultrasound (US)-guided core needle biopsies (CNBs) of breast lesions with at least 2 years of follow-up to determine the false-negative rate and to evaluate the diagnostic accuracy of CNB.
MethodsWe retrospectively analyzed 13,254 consecutive US-guided 14-gauge CNBs for breast lesions. We excluded biopsies if non-malignant biopsy result was not confirmed by surgical excision or US-guided vacuum-assisted biopsy, or fewer than 2 years of follow-up data were available. A total of 4,186 biopsies were excluded, and 9,068 breast masses from 7,039 women were included. The pathologic findings from each CNB were assessed using the standard diagnostic reference, defined based on the results of surgical excision, vacuum-assisted biopsy, or at least 2 years of long-term imaging follow-up. The false-negative rate and underestimation rate were calculated.
ResultsOf the 9,068 CNBs, benign pathology was found in 64.2%, high-risk results in 3.5%, and malignant results in 32.3%. Of the 5,821 benign CNBs, an additional malignancy was found at excision in 63 lesions, leading to a false-negative rate of 2.0% (63 of 3,067). The underestimation rate was 33.6% (111 of 330) for ductal carcinoma in situ and 24.5% (79 of 322) for high-risk results at surgical excision. Most false-negative diagnoses (84.1%, 53 of 63) were recognized through imaging-histology correlations, and immediate rebiopsies were performed. Ten malignancies (15.9%, 10 of 63) had delayed diagnoses and showed progression in follow-up US imaging.
Imaging-guided core needle biopsy (CNB) is becoming the standard of diagnosis for breast disease as a reliable alternative to open excision biopsy [1-5]. Percutaneous image-guided CNB is performed under either stereotactic or ultrasonographic guidance. Percutaneous ultrasound (US)-guided breast biopsy has several advantages over stereotactic or surgical biopsy, and many studies have reported CNB to be effective and safe. This procedure is safe; faster; less expensive; is performed in real time, allowing accurate assessments; and does not involve ionizing radiation [4,6,7].
However, the possibility of false-negative results is unavoidable; false-negatives can result from core needle sampling errors, failure to recognize imaging-histology discordances, and inappropriate follow-up periods for benign biopsies [8,9]. A delayed diagnosis of breast cancer is an outcome that every clinician strives to avoid. In previous studies of US-guided 14-gauge CNBs with at least 2 years of follow-up, the false-negative rates has ranged from 0.1% to 2.5% [5,10-12]. So far, some studies have been published on US-guided CNB for breast lesions with long-term follow-up periods [5,6,13,14], but most previous reports of US-guided CNB in the breast were based on a small number of biopsies. Furthermore, few studies have reported large series of US-guided CNB with long-term follow-up; therefore, this study was undertaken, based on a relatively large series of US-guided 14-gauge CNB procedures performed for breast lesions with long-term follow-up periods.
The purpose of this study was to assess the outcomes of US-guided CNB for breast lesions that had at least a 2-year follow-up for benign lesions in a large series to determine the false-negative rate and to evaluate the diagnostic performance of CNB.
This study was conducted with Institutional Review Board approval, and informed consent was waived because of the retrospective nature of this study.
From July 2005 to December 2012, 13,254 consecutive US-guided 14-gauge CNBs for breast lesions were performed at our institution. We retrospectively reviewed all the biopsy results and excluded 4,186 biopsies for which a non-malignant biopsy result was not confirmed by surgical excision or US-guided vacuum-assisted biopsy, or at least 2 years of follow-up data were not available.
A total of 9,068 breast masses in 7,039 women (age range, 11 to 92 years; mean, 46 years) were included in this study. Of the 7,039 patients, 1,253 had biopsies of two separate lesions, 234 had biopsies of three separate lesions, 74 had biopsies of four separate lesions, 11 had biopsies of five separate lesions, four had biopsies of six separate lesions, one had biopsies of seven separate lesions, one had biopsies of eight separate lesions, and one had biopsies of 10 separate lesions.
US-guided 14-gauge CNB was performed using a free-hand technique and a high-resolution US unit with 7.5- or 12-MHz linear array transducers (HDI 5000 or IU22, Philips-Advanced Technology Laboratories, Bothell, WA, USA; or Logiq 9, GE Healthcare, Milwaukee, WI, USA). Each procedure was performed in an outpatient setting under local anesthesia with the patient in the supine position. A 14-gauge automated core biopsy needle with a spring-loaded biopsy gun (Promac 2.2L, Manan Medical Products, Northbrook, IL, USA) or a 14-gauge dual-action semiautomatic core biopsy needle with a 22-mm throw (Stericut cut with coaxial, TSK Laboratory, Tochigi, Japan) was used. All biopsies were performed by one of 19 radiologists in fellowship training (n=15) or with extensive clinical experience (n=4) who were specialists in breast imaging and biopsies. According to our standard protocol, four or five core samples per lesion were routinely obtained.
For each lesion that underwent a CNB, a radiologist reviewed the pathology report in conjunction with the images obtained before, during, and after the biopsy procedure, and based on the results of the review, an addendum was attached to the biopsy report recommending specific management strategies for the patients and the referring physicians [15,16]. The imaging and histological findings were considered to be concordant when the histological findings provided a sufficient explanation for the imaging findings and discordant when they did not [15]. For malignant lesions (e.g., invasive carcinoma, ductal carcinoma in situ [DCIS], and metastases) identified through 14-gauge CNB, immediate definitive surgery or chemotherapy was recommended, in accordance with what was deemed clinically appropriate. For high-risk lesions (e.g., atypia including atypical ductal hyperplasia [ADH], lobular neoplasia, radial sclerosing lesions, and possible phyllodes tumors) and benign lesions (neither malignant nor high-risk) with imaging-histology discordance (i.e., a lesion that was thought to be malignant based on imaging but was demonstrated to be benign by histological findings) resulted in recommendations for surgical excision [15,17].
US follow-up at 6 months after biopsy and then annually for at least 2 years was recommended in patients with concordant benign lesions (i.e., a lesion that was suspected of being benign based on imaging and also proven to be benign by the histological findings). For some concordant benign lesions, rebiopsies were carried out by surgical excision or US-guided vacuum-assisted biopsy at the request by the patient or referring physician or because of suspicious physical findings (i.e., a palpable mass or nipple discharge) or lesion progression at US follow-up. The choice of surgical excision or US-guided vacuum-assisted biopsy at rebiopsy depended on the patient’s or referring physician’s preferences.
The pathologic findings of US-guided 14-gauge CNBs and rebiopsies, as well as the follow-up results, were obtained from patients’ medical records. After reviewing the results, pathologic comparisons between US-guided 14-gauge CNB results and the standard diagnostic reference were made. The standard diagnostic reference included the results of surgical excision, vacuum-assisted biopsy, or at least 2 years of long-term imaging follow-up. The false-negative rate was calculated as the proportion of benign results from US-guided 14-gauge CNBs among all breast cancers [5,10]. The underestimation rate for high-risk lesions was calculated as the proportion of lesions diagnosed as high-risk by CNB that were finally proven to be DCIS or invasive cancer after surgical excision. The underestimation rate for DCIS was likewise defined as the proportion of lesions diagnosed as DCIS by CNB that were finally proven to be invasive cancer after surgical excision [5]. For the false-negative results of US-guided 14-gauge CNB, the time interval between the CNB and rebiopsy, as well as the reasons for rebiopsy, were reviewed.
The lesions ranged in size from 2 to 130 mm (mean, 14.0 mm) as measured by US. The distribution of size and Breast Imaging-Reporting and Data System (BI-RADS) categories are summarized in Table 1. Of 9,068 breast masses, 4,408 lesions (48.6%) were less than 10 mm, and the most common indication for biopsy was the presence of a BI-RADS category 4a lesion (54.0%, 4,900 of 9,068). Three CNBs were BI-RADS category 1; among these, two cases were regarded as normal breast tissue on US, but the patient requested a biopsy. The remaining such case was a patient who had undergone a mastectomy and showed normal breast tissue on US, but a positron emission tomography scan revealed increased fluorodeoxyglucose uptake. All three cases were identified as benign on pathologic reports after the biopsies.
Among the 9,068 breast masses, the pathologic results of US-guided 14-gauge CNB were benign in 64.2% (5,821 lesions), high-risk in 3.5% (322 lesions), and malignant in 32.3% (330 DCIS lesions and 2,595 cases of invasive cancer) (Tables 2, 3). A total of 4,782 cases were confirmed by surgical excision or US-guided vacuum-assisted biopsy; in these cases, breast cancer was found in 3.2% (63 of 1,990) of benign CNBs, 26.3% (79 of 300) of high-risk CNBs, and 100% (2,492 of 2,492) in malignant CNBs at the final diagnosis. The malignancy rates were 0% (0 of 3) for lesions in BI-RADS category 1, 2.8% (1 of 35) in category 2, 2.2% (28 of 1,248) in category 3, 9.4% (463 of 4,900) in category 4a, 47.7% (267 of 560) in category 4b, 83.9% (640 of 762) in category 4c, and 97.8% (1,526 of 1,560) in category 5. The underestimation rate was 33.6% (111 of 330) for DCIS and 24.5% (79 of 322) for high-risk lesions at surgical excision (Fig. 1).
Of the 5,821 benign CNB results, 63 lesions were confirmed to be malignant after surgical excision (1.1%, 63 of 5,821). After postbiopsy review, 5,586 benign CNBs showed imaging-pathology concordance, which included 29 lesions that were ultimately diagnosed as malignant (0.5%, 29 of 5,586), while 235 benign CNBs showed imaging-pathology discordance (Fig. 2), including 34 lesions determined to be malignant after the subsequent surgical excision (14.4%, 34 of 235). A total of 3,067 cases were malignant at the final diagnosis, and the false-negative rate was 2.0% (63 of 3,067) with a sensitivity of 95.4% (2,927 of 3,067). Of the false-negative results, papillary lesions represented the highest percentage (36.5%, 23 of 63), followed by fibrocystic changes (20.6%, 13 of 63) and fibrosis (9.5%, 6 of 63) (Table 2). Most false-negative diagnoses (84.1%, 53 of 63) underwent immediate rebiopsy (interval range, 3 to 79 days; mean, 31.1 days) because the radiologist noted the discordance between the imaging findings and CNB and recommended surgical excision or a vacuum-assisted biopsy.
Ten malignancies (15.9%, 10 of 63) had delayed diagnoses, and the mean time interval between the initial CNB and excision was 11.6 months (range, 5 to 17 months) (Table 4). For five benign lesions, although radiologists performed imaging-pathology correlations and recommended surgical excision immediately after the initial CNB, the patients refused excision, resulting in delayed diagnoses of malignancy. All 10 delayed false-negative diagnoses showed progression in follow-up US imaging (Fig. 3). Of the five cases in which excision was refused, one lesion (Table 4, patient 1) was categorized as BI-RADS category 4a and the result of the initial US-guided CNB was fibrocystic change, which was considered to be concordant. However, a radiologist recommended surgical excision because of its large size (30 mm); the lesion increased in size from 30 to 43 mm over 17 months, and was confirmed as a malignant phyllodes tumor following surgical excision. Another patient (Table 4, patient 8) had a history of breast surgery due to intraductal papilloma 13 years ago, and a new mass was categorized as BI-RADS category 4a. The result of CNB was focal fibrosis, but a radiologist concluded that the lesion had high possibility of being intraductal papilloma and recommended surgical excision. The lesion markedly increased in size from 24 to 50 mm over 17 months, and was confirmed as invasive lobular carcinoma following surgical excision. Both of these patients refused excision after the initial CNB, causing delayed diagnoses of malignancy.
In this study, we analyzed a large population in which US-guided CNBs of breast lesions were performed from July 2005 to December 2012, building upon the previous study conducted from February 2000 through June 2005 at our institution [5]. The inclusion criteria were the same in both studies, and we included a total of 9,068 breast masses in this study, in contrast to 2,420 masses in the previous study. The mean lesion size was smaller in this study (mean, 14.0 mm; range, 2 to 130 mm) than in the previous study (mean, 18.7 mm; range, 2 to 180 mm), but we showed a lower false negative rate of 2.0% (63 of 3,067) than the false-negative rate of 2.4% (31 of 1,312) observed in the previous study. Overall, the results of our study showed that US-guided 14-gauge CNB for breast lesions exhibited better performance, based on the analysis of a larger population with smaller lesions in contrast to the sample of the preceding study from the same institution.
False-negative diagnoses are unavoidable for reasons such as sampling errors, failure to act upon imaging-histology discordances, or the absence of imaging follow-up after a benign biopsy [9]. However, most false-negative diagnoses can be immediately recognized through careful post-biopsy review, as was exemplified by our data. Most of our false-negative results (84.1%, 53 of 63) underwent immediate rebiopsies, avoiding delayed diagnoses of cancer, because imaging-pathology discordances were promptly detected by radiologists. Previous studies have shown similar results [5,6,13,18]. Several studies have suggested that physicians should perform rebiopsies or surgical excision for discordant CNBs because of the high prevalence of carcinoma in imaging-pathology discordant lesions [15,19,20]. Our data likewise showed a much higher malignant rate of 14.4% in discordant benign CNBs, which is within the 6.8%-24.4% range reported in previous studies [15,19,21], compared with a malignancy rate of 0.5% in concordant benign CNBs. These results accentuate the importance of imaging-pathology correlations after biopsy and the significance of discordant lesions.
Of the false-negative diagnoses, papillary lesions comprised the largest subgroup (36.5%, 23 of 63) in terms of the histological classification, and 3.1% (23 of 728) of the papillary lesions that underwent CNB were upgraded to malignancy; this is comparable with previous studies, in which 2.3%-14% of papillary lesions were upgraded to DCIS or invasive cancer after surgical excision [22-26]. To decide whether a papillary lesion is benign or malignant on the basis of CNB is challenging because of the heterogeneity of papillomas and targeting error [27,28]. It remains controversial whether all benign papillary lesions on CNB should undergo surgical excision to avoid false-negative diagnoses. Our data showed an upgrade rate to carcinoma of only 2.3% (16 of 686) in concordant papillomas, in contrast to an upgrade rate of 16.6% (7 of 42) in discordant papillomas; this supports the suggestions of recent reports that observation is sufficient, rather than surgical excision, for papillomas identified as benign by CNB if the imaging-pathology findings are concordant [26,29].
Ten malignancies had delayed diagnoses without immediate rebiopsies in the false-negative results. All these lesions showed progression in follow-up US. After review of the patients’ medical records, we found that patients refused excision for five false-negative lesions, although radiologists performed imaging-pathology correlations for these lesions and recommended surgical excision immediately after the initial CNB. The other five delayed false-negative diagnoses were identified as malignancies on follow-up sonography within 1 year after the initial CNB. Our results suggest that it is important to consider not only appropriate follow-up, but patient compliance after the biopsy. In previous studies, compliance rates of only 54% (49 of 90 lesions) [30] and 50.9% (84 of 165 lesions) [31] were reported for imaging surveillance recommendations. Therefore, poor patient compliance and inappropriate follow-up may cause further delays in the diagnosis and treatment of breast cancer, even if imaging-pathology discordances are recognized immediately.
One of the limitations of CNB is the histologic underestimation of breast malignancy, which means that lesions found to be high-risk or DCIS by a percutaneous breast biopsy are upgraded to DCIS or invasive cancer after surgical excision. In previous studies, the underestimation rates ranged from 6.25% to 65% for ADH and from 16% to 66% for DCIS using CNB or vacuum-assisted biopsy [13,32-35]. Our underestimation rates were 24.5% for high-risk lesions (79 of 322) and 33.6% for DCIS (111 of 330), comparable with previous reports.
Our study has a few limitations. First, there was no retrospective pathologic review of the core needle samples for the false-negative results. Second, the radiologists who performed the biopsies were heterogeneous, and included radiologists undergoing fellowship training (n=15). However, our institution is a tertiary hospital and it is natural for less experienced trainees to perform biopsies. Furthermore, our results showed reliable sensitivity (95.4%) and a low false-negative rate (2.0%).
In conclusion, US-guided 14-gauge CNB is accurate and provides optimal diagnostic information for breast lesions. Imaging-histology correlations and appropriate imaging follow-up should be performed to identify possible false-negative results and to avoid delayed diagnoses.
References1. Fuhrman GM, Cederbom GJ, Bolton JS, King TA, Duncan JL, Champaign JL, et al. Image-guided core-needle breast biopsy is an accurate technique to evaluate patients with nonpalpable imaging abnormalities. Ann Surg 1998;227:932–939.
2. Liberman L. Clinical management issues in percutaneous core breast biopsy. Radiol Clin North Am 2000;38:791–807.
3. Dillon MF, Hill AD, Quinn CM, O'Doherty A, McDermott EW, O'Higgins N. The accuracy of ultrasound, stereotactic, and clinical core biopsies in the diagnosis of breast cancer, with an analysis of false-negative cases. Ann Surg 2005;242:701–707.
4. Bassett LW, Mahoney MC, Apple SK. Interventional breast imaging: current procedures and assessing for concordance with pathology. Radiol Clin North Am 2007;45:881–894.
5. Youk JH, Kim EK, Kim MJ, Oh KK. Sonographically guided 14-gauge core needle biopsy of breast masses: a review of 2,420 cases with long-term follow-up. AJR Am J Roentgenol 2008;190:202–207.
6. Liberman L, Feng TL, Dershaw DD, Morris EA, Abramson AF. US-guided core breast biopsy: use and cost-effectiveness. Radiology 1998;208:717–723.
7. Philpotts LE, Hooley RJ, Lee CH. Comparison of automated versus vacuum-assisted biopsy methods for sonographically guided core biopsy of the breast. AJR Am J Roentgenol 2003;180:347–351.
8. Youk JH, Kim EK, Kim MJ, Lee JY, Oh KK. Missed breast cancers at US-guided core needle biopsy: how to reduce them. Radiographics 2007;27:79–94.
9. Perrot N, Jalaguier-Coudray A, Frey I, Thomassin-Naggara I, Chopier J. US-guided core needle biopsy: false-negatives: how to reduce them? Eur J Radiol 2013;82:424–426.
10. Youk JH, Kim EK, Kim MJ, Kwak JY, Son EJ. Analysis of false-negative results after US-guided 14-gauge core needle breast biopsy. Eur Radiol 2010;20:782–789.
11. Fajardo LL, Pisano ED, Caudry DJ, Gatsonis CA, Berg WA, Connolly J, et al. Stereotactic and sonographic large-core biopsy of nonpalpable breast lesions: results of the Radiologic Diagnostic Oncology Group V study. Acad Radiol 2004;11:293–308.
12. Zhang C, Lewis DR, Nasute P, Hayes M, Warren LJ, Gordon PB. The negative predictive value of ultrasound-guided 14-gauge core needle biopsy of breast masses: a validation study of 339 cases. Cancer Imaging 2012;12:488–496.
13. Crystal P, Koretz M, Shcharynsky S, Makarov V, Strano S. Accuracy of sonographically guided 14-gauge core-needle biopsy: results of 715 consecutive breast biopsies with at least two-year follow-up of benign lesions. J Clin Ultrasound 2005;33:47–52.
14. Smith DN, Rosenfield Darling ML, Meyer JE, Denison CM, Rose DI, Lester S, et al. The utility of ultrasonographically guided large-core needle biopsy: results from 500 consecutive breast biopsies. J Ultrasound Med 2001;20:43–49.
15. Liberman L, Drotman M, Morris EA, LaTrenta LR, Abramson AF, Zakowski MF, et al. Imaging-histologic discordance at percutaneous breast biopsy. Cancer 2000;89:2538–2546.
16. Parikh J, Tickman R. Image-guided tissue sampling: where radiology meets pathology. Breast J 2005;11:403–409.
17. Berg WA. Image-guided breast biopsy and management of high-risk lesions. Radiol Clin North Am 2004;42:935–946.
18. Schoonjans JM, Brem RF. Fourteen-gauge ultrasonographically guided large-core needle biopsy of breast masses. J Ultrasound Med 2001;20:967–972.
19. Kim MJ, Kim EK, Park SY, Jung HK, Park BW, Kim H, et al. Imaging-histologic discordance at sonographically guided percutaneous biopsy of breast lesions. Eur J Radiol 2008;65:163–169.
20. Soyder A, Taskin F, Ozbas S. Imaging-histological discordance after sonographically guided percutaneous breast core biopsy. Breast Care (Basel) 2015;10:33–37.
21. Son EJ, Kim EK, Youk JH, Kim MJ, Kwak JY, Choi SH. Imaging-histologic discordance after sonographically guided percutaneous breast biopsy: a prospective observational study. Ultrasound Med Biol 2011;37:1771–1778.
22. Jaffer S, Nagi C, Bleiweiss IJ. Excision is indicated for intraductal papilloma of the breast diagnosed on core needle biopsy. Cancer 2009;115:2837–2843.
23. Cheng TY, Chen CM, Lee MY, Lin KJ, Hung CF, Yang PS, et al. Risk factors associated with conversion from nonmalignant to malignant diagnosis after surgical excision of breast papillary lesions. Ann Surg Oncol 2009;16:3375–3379.
24. Mercado CL, Hamele-Bena D, Oken SM, Singer CI, Cangiarella J. Papillary lesions of the breast at percutaneous core-needle biopsy. Radiology 2006;238:801–808.
25. Sydnor MK, Wilson JD, Hijaz TA, Massey HD, Shaw de Paredes ES. Underestimation of the presence of breast carcinoma in papillary lesions initially diagnosed at core-needle biopsy. Radiology 2007;242:58–62.
26. Pareja F, Corben AD, Brennan SB, Murray MP, Bowser ZL, Jakate K, et al. Breast intraductal papillomas without atypia in radiologic-pathologic concordant core-needle biopsies: rate of upgrade to carcinoma at excision. Cancer 2016;122:2819–2827.
27. Ueng SH, Mezzetti T, Tavassoli FA. Papillary neoplasms of the breast: a review. Arch Pathol Lab Med 2009;133:893–907.
28. Philpotts LE. Percutaneous breast biopsy: emerging techniques and continuing controversies. Semin Roentgenol 2007;42:218–227.
29. Swapp RE, Glazebrook KN, Jones KN, Brandts HM, Reynolds C, Visscher DW, et al. Management of benign intraductal solitary papilloma diagnosed on core needle biopsy. Ann Surg Oncol 2013;20:1900–1905.
30. Goodman KA, Birdwell RL, Ikeda DM. Compliance with recommended follow-up after percutaneous breast core biopsy. AJR Am J Roentgenol 1998;170:89–92.
31. Pal S, Ikeda DM, Birdwell RL. Compliance with recommended follow-up after fine-needle aspiration biopsy of nonpalpable breast lesions: a retrospective study. Radiology 1996;201:71–74.
32. Kim H, Youk JH, Kim JA, Gweon HM, Jung WH, Son EJ. US-guided 14G core needle biopsy: comparison between underestimated and correctly diagnosed breast cancers. Asian Pac J Cancer Prev 2014;15:3179–3183.
33. Jang M, Cho N, Moon WK, Park JS, Seong MH, Park IA. Underestimation of atypical ductal hyperplasia at sonographically guided core biopsy of the breast. AJR Am J Roentgenol 2008;191:1347–1351.
Ductal carcinoma in situ in a 31-year-old woman.A, B. Transverse (A) and longitudinal (B) ultrasonography shows an oval microlobulated mass (arrows) categorized as BI-RADS category 4a. The result of ultrasound-guided core needle biopsy was atypical ductal hyperplasia, which was considered to be concordant. Surgical excision was recommended due to the high-risk pathology, and ductal carcinoma in situ was confirmed by excision. BI-RADS, Breast Imaging-Reporting and Data System.
 Fig. 1.Invasive ductal carcinoma in a 51-year-old woman.A, B. Transverse (A) and longitudinal (B) ultrasonography shows a microlobulated hypoechoic mass (arrows) categorized as BI-RADS category 4b. The result of ultrasound-guided core needle biopsy was fibrocystic change, which was considered to be discordant. Vacuum-assisted biopsy was recommended immediately and invasive ductal carcinoma was confirmed by vacuum-assisted biopsy.
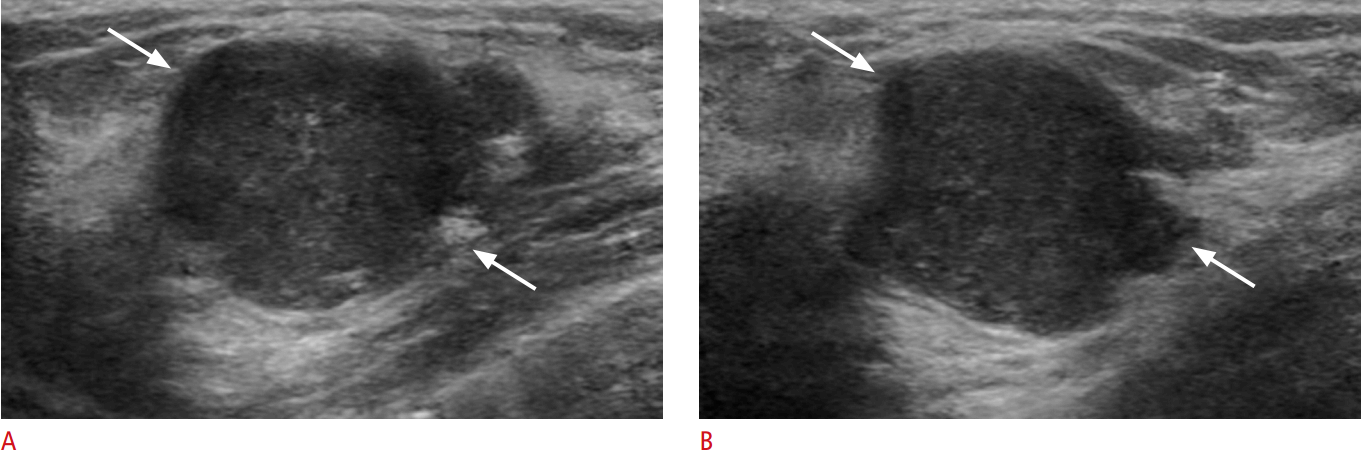 Fig. 2.Invasive lobular carcinoma in a 75-year-old woman.A, B. Ultrasonography shows a solid and cystic mass (arrows) (A) categorized as BI-RADS category 4a. The result of ultrasound-guided core needle biopsy was focal fibrosis. After 17 months, the mass increased in size from 24 to 50 mm (arrows) (B). Surgical excision was performed and invasive lobular carcinoma was confirmed.
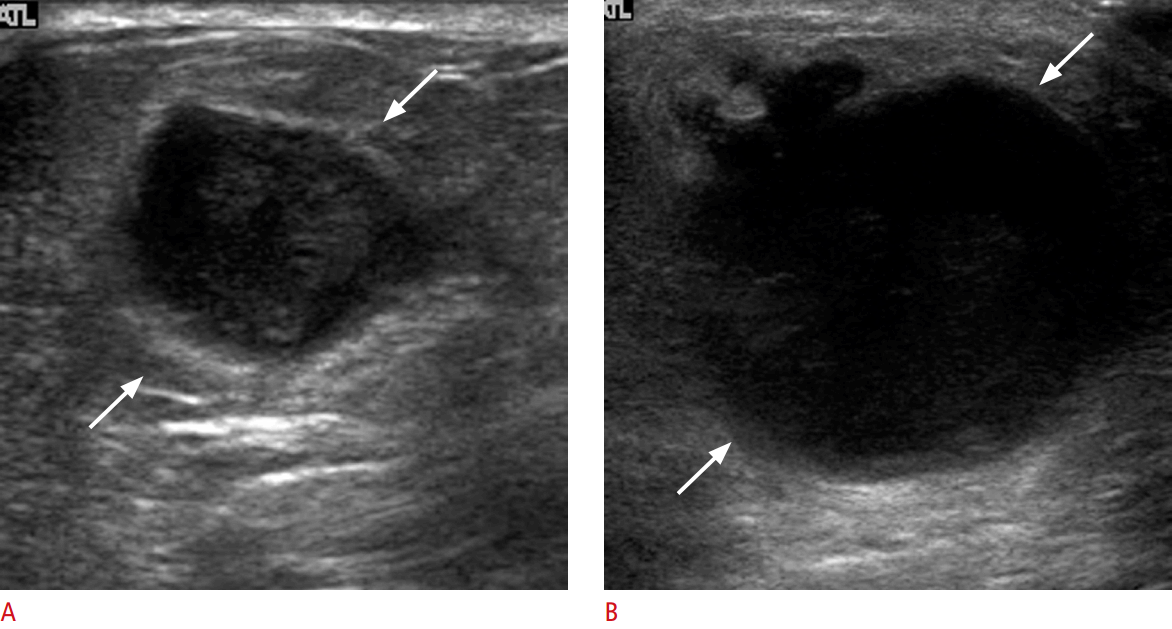 Fig. 3.Table 1.Distribution of the size and BI-RADS categories of lesions assessed using US-guided 14-gauge core needle biopsy Table 2.Pathological results of benign lesions on US-guided 14-gauge core needle biopsy correlated with the standard diagnostic reference Table 3.Pathological results of high-risk and malignant lesions on US-guided 14-gauge core needle biopsy correlated with the standard diagnostic reference Table 4.Delayed false-negative diagnoses after US-guided 14-gauge core needle biopsy
|



 Print
Print facebook
facebook twitter
twitter Linkedin
Linkedin google+
google+
 Download Citation
Download Citation PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC




