AbstractPurposeThis study was conducted to determine the malignancy risk and diagnostic value of various types of nonshadowing echogenic foci (NEF) in the risk stratification of thyroid nodules.
MethodsA total of 1,018 consecutive thyroid nodules (Ōēź1 cm) with final diagnoses were included. The presence of NEF was determined and types of NEF were classified according to the presence of a comet tail artifact (CTA), location, and size through a prospective evaluation. The associations with malignancy, malignancy risk, and diagnostic value of various types of NEF were assessed.
ResultsIntrasolid punctate NEF without CTA was the only type of NEF that was an independent predictor of malignancy (P<0.001). The malignancy risk of intrasolid punctate NEF without CTA was substantially higher in solid hypoechoic nodules than in isoechoic or nonsolid nodules (71.3% vs. 9.2%, P<0.001). In solid hypoechoic nodules, slightly increased sensitivity (70.8% vs. 67.9%) for malignancy and a similar malignancy risk (71.4% vs. 71.3%) were observed for intrasolid punctate NEF (with or without CTA) and intrasolid punctate NEF without CTA, respectively. NEF with CTA at the margin of the cystic component was not associated with malignancy or benignity in nonsolid nodules (P>0.05).
ConclusionIntrasolid punctate NEF without CTA was the only independent predictor of malignancy. However, solid hypoechoic nodules with intrasolid punctate NEF should be classified as high-suspicion nodules regardless of coexisting CTA. Other types of NEF had no added value for detecting malignancy compared to intrasolid punctate NEF without CTA.
IntroductionUltrasonography (US) plays an increasingly essential role in assessments of the malignancy risk of thyroid nodules, the decision to perform fine-needle aspiration (FNA), and management decisions after FNA. The US features considered as consistent predictors of thyroid malignancy include solid composition, hypoechogenicity, microcalcifications, nonparallel orientation (taller than wide shape), and a spiculated/microlobulated (irregular) margin [1-4]. The echogenic foci detected in thyroid nodules have variable US features and are categorized as echogenic foci with and without posterior acoustic shadowing. Echogenic foci with posterior acoustic shadowing mostly correspond to macrocalcifications, which include intranodular or isolated macrocalcifications and rim calcification [5-7]. Although many studies [8-10] have investigated the diagnostic value of macrocalcifications, the majority of studies on nonshadowing echogenic foci (NEF) have focused on microcalcifications. The US lexicon of microcalcifications primarily defines them as punctate (Ōēż1 mm) echogenic foci with focal hyperechogenicity relative to the thyroid tissue within the solid component of a nodule [5,6]. Although the US feature of microcalcification is strongly associated with malignancy, some reports [7,11] have indicated that the term "microcalcification" is a misnomer because many punctate echogenic foci are found in benign nodules [12] and are not well correlated with psammomatous microcalcifications on histopathologic examinations [11,13,14]. Recent studies [12,15] have suggested that some types of NEF with a comet tail artifact (CTA) may be associated with malignancy, but this possibility remains controversial. Although several studies [12,15-18] have investigated the clinical significance of NEF with or without CTA, the malignancy risk and diagnostic value of various types of NEF have not been established. Therefore, this study aimed to determine the malignancy risk and diagnostic value of various types of NEF for the risk stratification of thyroid nodules.
Materials and MethodsThis retrospective study was approved by our Institutional Review Board, and the requirement for patients' informed consent was waived.
Study PopulationBetween March 2017 and November 2018, US-guided FNA or core needle biopsy (CNB) was performed for 1,468 consecutive thyroid nodules, including 233 nodules with a size <1 cm and 1,235 nodules with a size Ōēź1 cm. Among the 1235 nodules measuring Ōēź1 cm, 217 nodules without a final diagnosis confirmed by surgery or biopsy (FNA or CNB) were excluded from this study. Finally, 1,018 nodules (Ōēź1 cm) with final diagnoses in 832 patients were included in our study (679 women and 153 men; median age, 57 years; interquartile range [IQR], 48 to 64 years) (Fig. 1). Malignant nodules (n=145) were conclusively diagnosed on the basis of histopathologic results after surgery (n=107) or malignant FNA or CNB results (n=38). Benign nodules (n=873) were conclusively diagnosed according to histopathologic results after surgery (n=74), at least two benign FNA or CNB results (n=240), or one benign FNA or CNB result (n=559). In nodules with final diagnoses made by FNA or CNB, the final diagnosis of malignancy or benignity was made when the FNA or CNB result was malignant (category 6) or benign (category 2) according to the six categories of the Bethesda system [19] and the histologic diagnosis of the CNB specimen [20].
US Examination and Image AnalysisAll US examinations were performed using a 12-MHz linear probe and real-time US system (EPIQ7, Philips Healthcare, Bothell, WA, USA). The US features of thyroid nodules were prospectively evaluated before US-guided biopsy by one experienced radiologist (D.G.N.) with 21 years' experience in performing thyroid US and interventional procedures. The presence of NEF and its US characteristics were prospectively assessed by the interpreter with a predefined study protocol, and the other US features of composition, echogenicity, margin, orientation, shape, macrocalcifications or rim calcification, and spongiform appearance were also assessed by the interpreter [5]. The presence of NEF was defined as the observation of any obvious hyperechogenic foci with brighter echogenicity than that of the normal thyroid gland and no accompanying posterior shadowing. Ambiguous echogenic foci were not considered to indicate the presence of NEF, and echogenic foci with posterior shadowing including macrocalcifications and those with rim calcification, with or without posterior shadowing, were not considered to be NEF. Intracystic NEF was defined based on the presence of obvious echogenic foci within the cystic component, and the presence of CTA was defined as an obvious posterior echogenic tail accompanying NEF.
In the study protocol, the assessment of the location (intrasolid, margin of the cystic component, or intracystic), size (punctate [Ōēż1 mm] or large [>1 mm]), and the presence of accompanying CTA in the NEF were included. The location of NEF was classified as intrasolid or intracystic based on whether it was entirely located within the solid component or within the cystic content of a nodule (Figs. 2-6). The location of NEF abutting the wall or the septa of the cystic component was categorized as the margin of the cystic component. The location of NEF with CTA was determined according to the location of the head part of the NEF regardless of the location of the tail part. The size (punctate or large) of NEF without CTA was prospectively determined by a visual assessment of the maximal size of the NEF per nodule, and the measurement of the size of the NEF was allowed in some cases of small NEF when a visual judgement was difficult.
In NEF with CTA, the size of the head part was used to classify the NEF as punctate or large. The size of the head and tail of the NEF and the shape of CTA were retrospectively measured and assessed by the same interpreter who was blinded to the results of biopsy or surgery. The head or tail size of each NEF with CTA was obtained from the maximal head or tail size in the transverse or longitudinal image for each nodule. The head size was defined as the maximal transverse or longitudinal diameter of the head portion of the NEF, and the tail size of CTA was defined as the maximal anteroposterior diameter (upper margin of the head to the lower end of the tail) in each NEF with CTA. The shape of CTA was categorized as inverted triangular or linear. The types of NEF were categorized according to the combined findings of CTA (present or not), location (intrasolid, margin of the cystic component, or intracystic), and size (punctate or large) (Figs. 2-6). Large NEF located at the margin or septa of nonsolid nodules were further categorized as large linear and large nodular NEF. When a single nodule had multiple types of NEF, each type of NEF was recorded independently for each thyroid nodule.
Data Analysis and StatisticsContinuous variables are presented using the mean┬▒standard deviation or the median and interquartile range (IQR), according to whether each variable had a parametric or nonparametric distribution, respectively. Categorical variables are reported as frequencies and percentages for each category. The malignancy risk of various NEF types and the associations of NEF types with malignancy were assessed overall and in subgroups according to the composition and echogenicity of the nodules. The chi-square test or Fisher exact test was used to determine the significance of associations between NEF types and malignancy. Multivariable logistic regression analysis was performed to determine statistically significant independent US predictors among the various NEF types (P<0.05). The Mann-Whitney U test was used to compare the head and tail size of NEF with CTA between benign and malignant nodules. The chi-square test or Fisher exact test was used to compare the shape of NEF with CTA between benign and malignant nodules.
The diagnostic performance of intrasolid punctate NEF for detecting thyroid malignancy was evaluated according to the sensitivity, specificity, positive predictive value (PPV), negative predictive value, and accuracy in all nodules and subgroups. The chi-square test or Fisher exact test was used to compare the PPV of intrasolid punctate NEF between the subgroups according to the composition and echogenicity of nodules. Statistical analyses were performed using SPSS for Windows version 23.0 (IBM Corp., Armonk, NY, USA). A significant difference was defined as a P-value of <0.05.
ResultsClinical DataThe median size (maximal diameter) of the thyroid nodules was 19 mm (IQR, 14 to 28 mm). In the final diagnoses of 1,018 nodules, 873 (85.8%) were benign (849 benign non-neoplastic nodules, 24 follicular adenomas) and 145 (14.2%) were malignant, of which 127 (87.6%) were papillary thyroid carcinomas, 10 (6.9%) were follicular thyroid carcinomas, four (2.8%) were anaplastic carcinomas, three (2.1%) were metastases, and one (0.7%) was medullary carcinoma.
Associations of NEF with Malignancy According to the Presence of a CTA, Location, and SizeThe associations of NEF with malignancy according to the presence of an accompanying CTA, location, and size are presented in Table 1. NEFs of any type were found in 568 of the 1,018 nodules (55.8%), and NEFs were more frequently found in malignant tumors than in benign nodules (P<0.001) (Table 1). In the univariable analysis, the absence of CTA, intrasolid location, and punctate size of the NEF all showed significant associations with malignancy (all, P<0.001); however, in the multivariable analysis, only an intrasolid location was independently associated with malignancy (odds ratio [OR], 8.038; 95% confidence interval [CI], 3.610 to 17.901; P<0.001). Location of the NEF at the margin of the cystic component or in the intracystic content showed significant associations with benignity (P<0.001).
Associations of NEF Accompanied by CTA with Malignancy According to Size, Shape, and LocationIn 152 nodules with NEF accompanied by CTA, the malignancy rate showed no significant relationships with the head or tail size (small [Ōēż1 mm] vs. large [>1 mm]) regardless of the location of the NEF with CTA (P>0.05). No significant difference was found in the mean maximal head and tail size per nodule according to the location of the NEF between benign and malignant nodules (P>0.05). Although the head size of NEFs accompanied by CTA did not significantly differ according to location (P>0.05), the tail size of NEFs with CTA located at the margin of the cystic component or within the cystic content was significantly larger than that of intrasolid NEFs with CTA (P=0.006).
No significant difference was found in malignancy risk between linear and triangular CTAs according to the location of NEFs with CTA (P>0.05). However, the malignancy risk of nodules containing an intrasolid NEF with CTA was significantly higher than that of nodules with NEF accompanied by CTA located only at the margin or within the cystic component (23.2% [19 of 82] vs. 2.9% [2 of 70], P<0.001).
Associations of Various NEF Types with Malignancy and Malignancy Risk in Overall NodulesThe associations of various types of NEF with malignancy and the malignancy risk of NEF in the overall sample of nodules are shown in Table 2. A significant association with malignancy was observed for punctate intrasolid NEF with or without CTA and for large NEF without CTA within the solid component or at the margin of the cystic component (P<0.05). Among these types of NEF, only intrasolid punctate NEF without CTA was independently associated with malignancy in the multivariable analysis (OR, 4.101; 95% CI, 2.773 to 6.063; P<0.001) (Table 2). Among all the US features found to be associated with malignancy through a frequency analysis, independent associations with malignancy were found for solid composition, hypoechogenicity, spiculated/microlobulated margin, nonparallel orientation (taller than wide), macrocalcifications, and intrasolid punctate NEF without CTA through a multivariable analysis of the entire sample of nodules (all, P<0.001).
Among the 386 nodules with intrasolid punctate NEF, intrasolid punctate NEF without CTA was found in 315 nodules (81.6%), intrasolid punctate NEF partially accompanied by CTA in 58 nodules (15%), and intrasolid punctate NEF consistently accompanied by CTA in 13 nodules (3.4%). In nodules with intrasolid punctate NEF, there was no significant difference in malignancy risk among nodules without CTA (26.3%), those partially accompanied by CTA (24.1%), and those consistently accompanied by CTA (23.1%) (P>0.05). Intrasolid punctate NEF was more frequently found (66.7%-83.3%) in nodules with large NEF within the solid component or at the margin of the cystic component than in nodules without large NEF, regardless of the presence of accompanying CTA (32.9%-36.4%) (P<0.05).
Associations with Malignancy and Malignancy Risk of Various NEF Types According to Composition and EchogenicityThe associations of various types of NEF with malignancy and malignancy risk according to the composition and echogenicity of nodules are shown in Table 3. In the subgroup of solid hypoechoic nodules, intrasolid punctate NEF without CTA was found to be the only independent predictor of malignancy in the multivariable analysis (OR, 7.016; 95% CI, 3.853 to 12.775; P<0.001), and the malignancy risk of intrasolid punctate NEF without CTA was 71.3%. In solid hypoechoic nodules, except for those with intrasolid punctate NEF without CTA, the malignancy risk was 24.5%, and only intrasolid punctate NEF with CTA was a predictor of malignancy (malignancy risk, 75.0%) (P<0.05).
In the subgroup of solid isoechoic nodules, only intrasolid punctate NEF without CTA was associated with malignancy (P>0.05) and the malignancy risk was 13.2%. In the subgroup of nonsolid nodules, intrasolid punctate or large NEF without CTA and large nodular NEF without CTA at the margin of the cystic component were found to be independently associated with malignancy in the multivariable analysis (P<0.05).
Diagnostic Value of NEF for Malignancy Risk StratificationIntrasolid punctate NEF without CTA showed a significantly higher sensitivity for malignancy (66.9% vs. 11.7%, P<0.001) and a similar PPV in all nodules compared with the intrasolid punctate NEF with CTA (Table 4). In the subgroup of solid hypoechoic nodules, intrasolid punctate NEF with or without CTA slightly increased the sensitivity (2.1%) for malignancy compared to intrasolid punctate NEF without CTA, while maintaining almost the same PPV for malignancy. However, intrasolid punctate NEF with or without CTA did not increase the sensitivity or PPV compared to intrasolid punctate NEF without CTA alone in the subgroup of isoechoic or nonsolid nodules. The PPV of intrasolid punctate NEF without CTA was substantially higher in solid hypoechoic nodules than in isoechoic or nonsolid nodules (71.3% vs. 9.2%, P<0.001).
In eight (5.5%) malignant tumors, other types of NEF with no coexisting intrasolid punctate NEF without CTA were observed: three solid hypoechoic nodules with intrasolid punctate NEF accompanied by CTA, four nonsolid nodules with punctate or linear NEF not accompanied by CTA at the margin of the cystic component, and one nonsolid nodule with intracystic NEF partially accompanied by CTA. Among the other types of NEF that were predictive of malignancy, additional value for detecting malignant tumors was only shown by intrasolid punctate NEF with CTA in solid hypoechoic nodules that did not also have intrasolid punctate NEF without CTA. The malignancy risk of nodules that had intrasolid punctate NEF with CTA, but no concurrent intrasolid punctate NEF without CTA, was 23.1% (3 of 13) among all nodules and 75% (3 of 4) among solid hypoechoic nodules, which was similar to the malignancy risk of nodules with intrasolid punctate NEF without CTA (Table 4).
In the subgroup of solid hypoechoic and solid isoechoic nodules, the coexistence of other types of NEF predictive of malignancy did not significantly increase the malignancy risk of nodules with intrasolid punctate NEF without CTA (P>0.05). Meanwhile, in the subgroup of nonsolid nodules, the coexistence of large NEF without CTA located within the solid component and at the margin of the cystic component significantly increased the malignancy risk of nodules with intrasolid punctate NEF without CTA (24.4%, and 60%, P<0.05) (Fig. 4). NEF with CTA located at the margin or within the cystic component was not significantly predictive of malignancy or benignity in nonsolid nodules (P>0.05). Although no type of NEF significantly decreased the malignancy risk in the subgroup of nonsolid nodules, intracystic NEF with CTA showed the lowest malignancy risk (2.2%, 1 of 46).
DiscussionIn this study, we investigated the malignancy risk of various types of NEF and the diagnostic value of NEF for malignancy risk stratification of thyroid nodules. Our results showed that among various types of NEF, only intrasolid punctate NEF without CTA was a reliable independent predictor of malignancy. Meanwhile, the malignancy risk of intrasolid punctate NEF without CTA was substantially different according to the combination of nodule composition and echogenicity, which supports the result of a previous study indicating that the malignancy risk of intrasolid punctate echogenic foci (microcalcifications) should be stratified according to the composition and echogenicity of the nodules [4].
The prevalence of intrasolid punctate NEF without CTA considered as microcalcifications was substantially higher (31.6%) than the prevalence (7.6%-8.4%) of microcalcifications in benign nodules and slightly higher (66.9%) than the prevalence (41.6%-51.4%) of microcalcifications in malignant nodules according to cohort data on thyroid nodules (Ōēź1 cm) from previous studies [4,9]. This discrepancy may be due to differences in study design among studies. We prospectively interpreted US images during real-time US imaging with a specific study protocol focused on NEF, which enabled us to detect more punctate NEF than would be possible through a retrospective evaluation of limited static images obtained without a specific protocol.
NEF with CTA has been considered as a US feature of colloid material that is mostly found in benign cystic thyroid nodules [16]. Although several studies [15,18,21,22] have proposed an association between NEF with CTA with malignancy, the malignancy risk and diagnostic value of various types of NEF with CTA have not previously been investigated. Our study showed that the malignancy risk of NEF with CTA varied according to the location of NEF and US patterns of composition and echogenicity of nodules. First, intrasolid NEF with CTA increased the malignancy risk of solid hypoechoic and nonsolid nodules; however, NEF with CTA located at the margin of the cystic component was not predictive of malignancy or benignity in nonsolid nodules. Second, intrasolid NEF with CTA showed a substantially higher malignancy rate in solid hypoechoic nodules than in solid isoechoic or nonsolid nodules. Third, intrasolid punctate NEF with CTA showed a similarly high malignancy risk and slightly increased sensitivity for malignancy compared with intrasolid punctate NEF without CTA only in solid hypoechoic nodules, which suggests that solid hypoechoic nodules with intrasolid punctate NEF, regardless of the presence of CTA, should be categorized as high-suspicion nodules.
In our study, the calculated malignancy risk of intracystic NEF with CTA was slightly higher than 1%. However, selection bias was a relevant factor regarding this finding due to the exclusion of many benign nodules with intracystic CTA in our cohort data, as biopsy was not performed for most of these nodules. The collective data of nodules with intracystic CTA [4,15-17] indicate that nodules with intracystic CTA may have an actual malignancy risk of <1% and can be categorized as benign nodules.
Our study showed that an intrasolid location of NEF with CTA was associated with an increased risk of malignancy; however, the head or tail size of NEF with CTA was not significantly associated with malignancy. This finding is different from the result of a previous study [12], which reported that the malignancy rate was significantly lower in nodules with NEF with CTA with a large tail than in those with CTA with a small tail. The estimated malignancy rate could be lower in nodules with CTA with a large tail if in most benign nodules with CTA, the CTA is located inside the cyst or at the margin of the cystic component, because those locations are associated with a larger tail size than is the case for intrasolid NEF with CTA.
The histopathologic features of the various types of NEF remain unclear. Intracystic CTA is strongly correlated with inspissated colloid content [16]. However, many cases of punctate NEF may correspond to dystrophic calcifications and colloid, as well as psammomatous calcifications [11,14,23] and it remains unclear whether the histopathologic feature of intrasolid punctate NEF with CTA is colloid or microcalcification [21,22,24]. Although the histopathologic features of large NEFs remain to be elucidated, they might have a relationship with those of intrasolid punctate NEFs without CTA based on the high rate of their concurrence.
Our study has several limitations. First, there may have been selection bias because we excluded some patients without a final diagnosis and included only thyroid nodules in which US-guided biopsy was performed. Second, the final diagnosis was based on the results of biopsy and histology of surgical specimens, which has an inherent risk of false-negative or false-positive results. Third, only one experienced radiologist prospectively interpreted the US features of thyroid nodules and we could not assess interobserver agreement regarding NEF. Further studies may be required to verify the reproducibility of our results. Fourth, the histologic features of various NEFs were not documented. Further studies investigating the histopathologic features of various types of NEF in thyroid nodules are needed.
In conclusion, intrasolid punctate NEF without CTA was the only independent predictor of malignancy among the various types of NEF, and this finding posed a substantially higher malignancy risk in solid hypoechoic nodules than in isoechoic or nonsolid nodules. Intrasolid punctate NEF with or without CTA showed a similarly high malignancy risk and slightly increased sensitivity for detecting malignancy compared to intrasolid punctate NEF without CTA in solid hypoechoic nodules. Therefore, solid hypoechoic nodules with intrasolid punctate NEF should be classified as high-suspicion nodules regardless of the presence of CTA. NEF with CTA located at the margin or within the cystic component was not predictive of malignancy or benignity in nonsolid nodules. Other types of NEF had no added value for detection of malignancy compared with intrasolid punctate NEF without CTA. The most important type of NEF is intrasolid punctate NEF, and other types of NEF may not be useful in the risk stratification of thyroid nodules.
NotesAuthor Contributions Conceptualization: Na DG. Data acquisition: Na DG, Paik W, Gwon HY, Noh BJ. Data analysis or interpretation: Na DG, Sohn YM. Drafting of the manuscript: Sohn YM, Na DG. Critical revision of the manuscript: Na DG, Sohn YM. Approval of the final version of the manuscript: all authors. AcknowledgementsThis research was supported by the Medical Research Promotion Program through the Gangneung Asan Hospital funded by the Asan Foundation (2018-C03).
References1. Brito JP, Gionfriddo MR, Al Nofal A, Boehmer KR, Leppin AL, Reading C, et al. The accuracy of thyroid nodule ultrasound to predict thyroid cancer: systematic review and meta-analysis. J Clin Endocrinol Metab 2014;99:1253ŌĆō1263.
2. Campanella P, Ianni F, Rota CA, Corsello SM, Pontecorvi A. Quantification of cancer risk of each clinical and ultrasonographic suspicious feature of thyroid nodules: a systematic review and meta-analysis. Eur J Endocrinol 2014;170:R203ŌĆōR211.
3. Remonti LR, Kramer CK, Leitao CB, Pinto LC, Gross JL. Thyroid ultrasound features and risk of carcinoma: a systematic review and meta-analysis of observational studies. Thyroid 2015;25:538ŌĆō550.
4. Na DG, Baek JH, Sung JY, Kim JH, Kim JK, Choi YJ, et al. Thyroid Imaging Reporting and Data System risk stratification of thyroid nodules: categorization based on solidity and echogenicity. Thyroid 2016;26:562ŌĆō572.
5. Shin JH, Baek JH, Chung J, Ha EJ, Kim JH, Lee YH, et al. Ultrasonography diagnosis and imaging-based management of thyroid nodules: revised Korean Society of Thyroid Radiology consensus statement and recommendations. Korean J Radiol 2016;17:370ŌĆō395.
6. Russ G, Bonnema SJ, Erdogan MF, Durante C, Ngu R, Leenhardt L. European Thyroid Association guidelines for ultrasound malignancy risk stratification of thyroid nodules in adults: the EU-TIRADS. Eur Thyroid J 2017;6:225ŌĆō237.
7. Grant EG, Tessler FN, Hoang JK, Langer JE, Beland MD, Berland LL, et al. Thyroid ultrasound reporting Lexicon: white paper of the ACR Thyroid Imaging, Reporting and Data System (TIRADS) Committee. J Am Coll Radiol 2015;12:1272ŌĆō1279.
8. Frates MC, Benson CB, Doubilet PM, Kunreuther E, Contreras M, Cibas ES, et al. Prevalence and distribution of carcinoma in patients with solitary and multiple thyroid nodules on sonography. J Clin Endocrinol Metab 2006;91:3411ŌĆō3417.
9. Moon WJ, Jung SL, Lee JH, Na DG, Baek JH, Lee YH, et al. Benign and malignant thyroid nodules: US differentiation: multicenter retrospective study. Radiology 2008;247:762ŌĆō770.
10. Malhi HS, Velez E, Kazmierski B, Gulati M, Deurdulian C, Cen SY, et al. Peripheral thyroid nodule calcifications on sonography: evaluation of malignant potential. AJR Am J Roentgenol 2019;213:672ŌĆō675.
11. Tahvildari AM, Pan L, Kong CS, Desser T. Sonographic-pathologic correlation for punctate echogenic reflectors in papillary thyroid carcinoma: what are they? J Ultrasound Med 2016;35:1645ŌĆō1652.
12. Malhi H, Beland MD, Cen SY, Allgood E, Daley K, Martin SE, et al. Echogenic foci in thyroid nodules: significance of posterior acoustic artifacts. AJR Am J Roentgenol 2014;203:1310ŌĆō1316.
13. Bilici S, Yigit O, Onur F, Hamit B, Nazli MA, Gunver F, et al. Histopathological investigation of intranodular echogenic foci detected by thyroid ultrasonography. Am J Otolaryngol 2017;38:608ŌĆō613.
14. Erdem Toslak I, Martin B, Barkan GA, Kilic AI, Lim-Dunham JE. Patterns of sonographically detectable echogenic foci in pediatric thyroid carcinoma with corresponding histopathology: an observational study. AJNR Am J Neuroradiol 2018;39:156ŌĆō161.
15. Wu H, Zhang B, Li J, Liu Q, Zhao T. Echogenic foci with comet-tail artifact in resected thyroid nodules: not an absolute predictor of benign disease. PLoS One 2018;13:e0191505.
16. Ahuja A, Chick W, King W, Metreweli C. Clinical significance of the comet-tail artifact in thyroid ultrasound. J Clin Ultrasound 1996;24:129ŌĆō133.
17. Beland MD, Kwon L, Delellis RA, Cronan JJ, Grant EG. Nonshadowing echogenic foci in thyroid nodules: are certain appearances enough to avoid thyroid biopsy? J Ultrasound Med 2011;30:753ŌĆō760.
18. Ha SM, Chung YJ, Ahn HS, Baek JH, Park SB. Echogenic foci in thyroid nodules: diagnostic performance with combination of TIRADS and echogenic foci. BMC Med Imaging 2019;19:28.
19. Cibas ES, Ali SZ. The Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2009;19:1159ŌĆō1165.
20. Jung CK, Min HS, Park HJ, Song DE, Kim JH, Park SY, et al. Pathology reporting of thyroid core needle biopsy: a proposal of the Korean Endocrine Pathology Thyroid Core Needle Biopsy Study Group. J Pathol Transl Med 2015;49:288ŌĆō299.
21. Patel BN, Kamaya A, Desser TS. Pitfalls in sonographic evaluation of thyroid abnormalities. Semin Ultrasound CT MR 2013;34:226ŌĆō235.
22. Klang K, Kamaya A, Tahvildari AM, Jeffrey RB, Desser TS. Atypical thyroid cancers on sonography. Ultrasound Q 2015;31:69ŌĆō74.
Flow diagram of patient enrollment.US, ultrasonography; FNA, fine-needle aspiration; CNB, core needle biopsy.
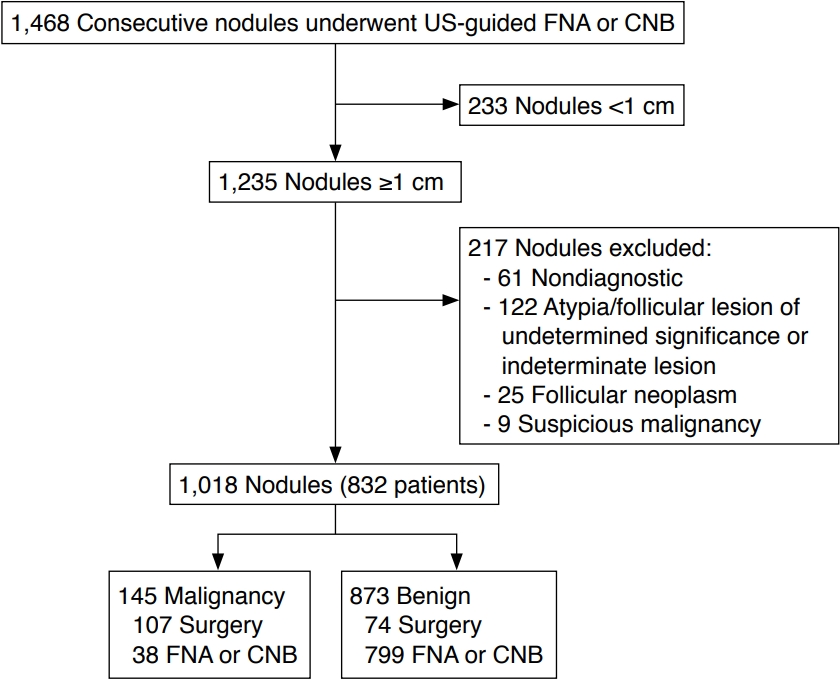 Fig.┬Ā1.Intrasolid punctate and large nonshadowing echogenic foci in solid hypoechoic nodules of papillary carcinoma.A. Transverse ultrasonography shows a solid hypoechoic nodule with intrasolid punctate nonshadowing echogenic foci without a comet tail artifact (arrow) and with a triangular comet tail artifact (tail size, 0.7 mm) (open arrow). B. Transverse ultrasonography shows a solid hypoechoic nodule with intrasolid large nonshadowing echogenic foci (open arrow) and intrasolid punctate nonshadowing echogenic foci without a comet tail artifact (arrow).
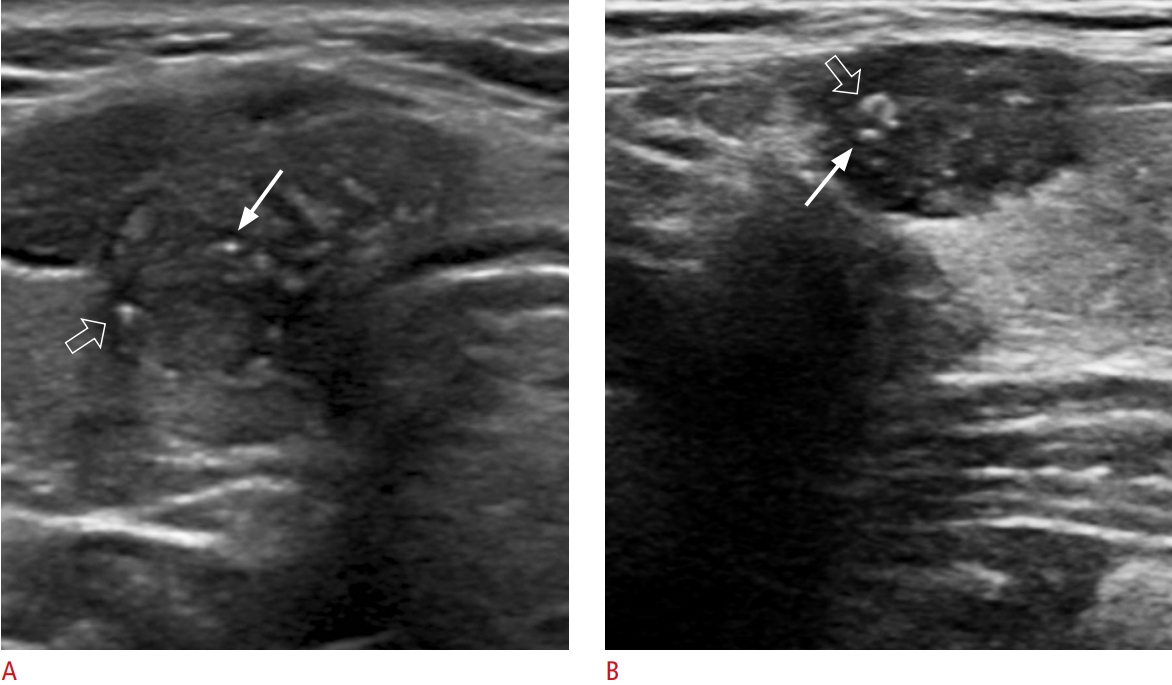 Fig.┬Ā2.Intrasolid punctate nonshadowing echogenic foci in solid isoechoic nodules of papillary carcinoma.Longitudinal ultrasonography shows a solid isoechoic nodule with intrasolid punctate nonshadowing echogenic foci without a comet tail artifact (arrow) and a nonparallel orientation (taller than wide).
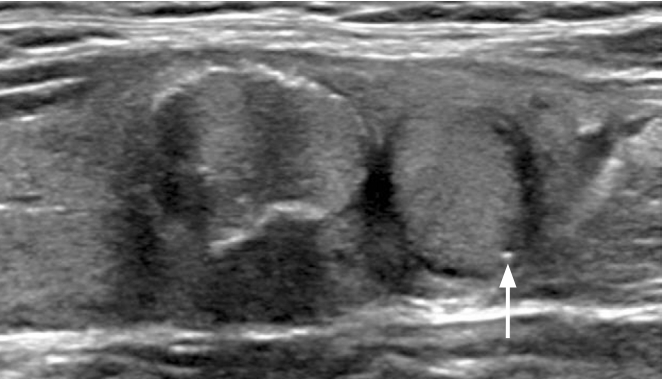 Fig.┬Ā3.Nonshadowing echogenic foci within the solid component and at the margin of cystic component in partially cystic nodules of papillary carcinoma.A. Longitudinal ultrasonography shows a partially cystic isoechoic nodule with intrasolid punctate nonshadowing echogenic foci without a comet tail artifact (arrow), and large nonshadowing echogenic foci without a comet tail artifact (arrowhead) and punctate nonshadowing echogenic foci with linear comet tail artifacts (tail size: 2.2 mm, 1.4 mm) (open arrows) at the margin of cystic component. B. Transverse ultrasonography shows a partially cystic hypoechoic nodule with intrasolid punctate nonshadowing echogenic foci (arrow) and multiple large nonshadowing echogenic foci without a comet tail artifact within the solid component and at the margin of the cystic component. There was a hypoechoic solid component in this nodule (not shown).
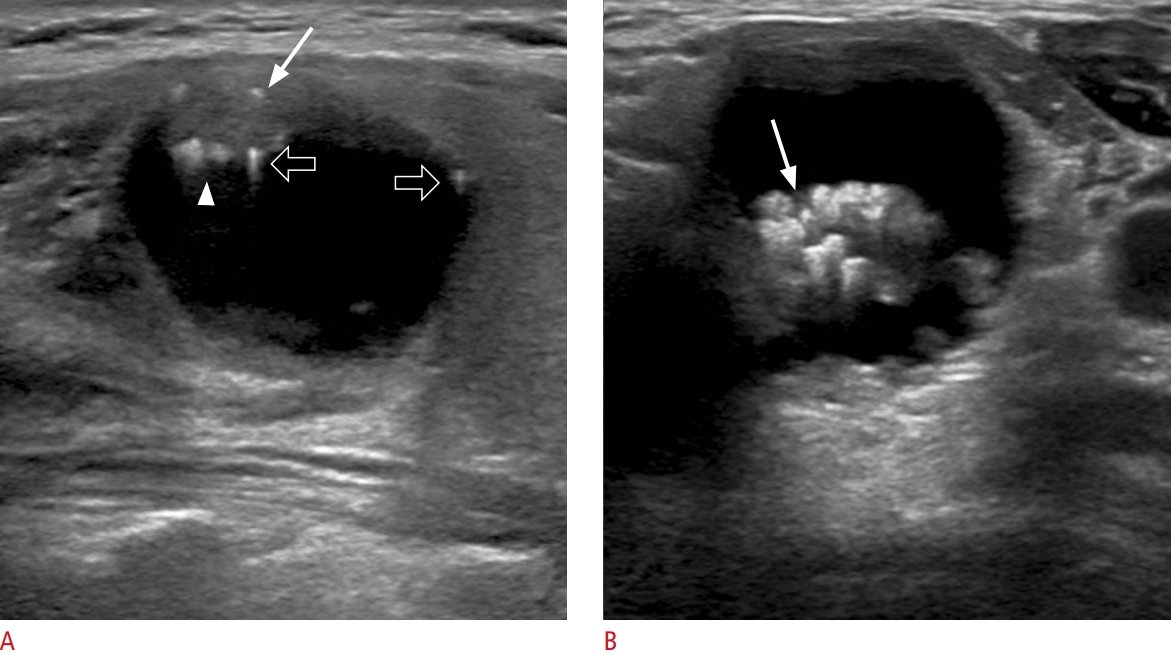 Fig.┬Ā4.Nonshadowing echogenic foci at the septa and margin of the cystic component in partially cystic nodules of minimally invasive follicular thyroid cancer.A. Transverse ultrasonography shows a partially cystic mixed echoic nodule with large linear nonshadowing echogenic foci without a comet tail artifact (arrow) at the septa of the cystic component. B. Transverse ultrasonography shows a predominantly solid isoechoic nodule with punctate nonshadowing echogenic foci without a comet tail artifact at the margin of the small cystic component (arrow).
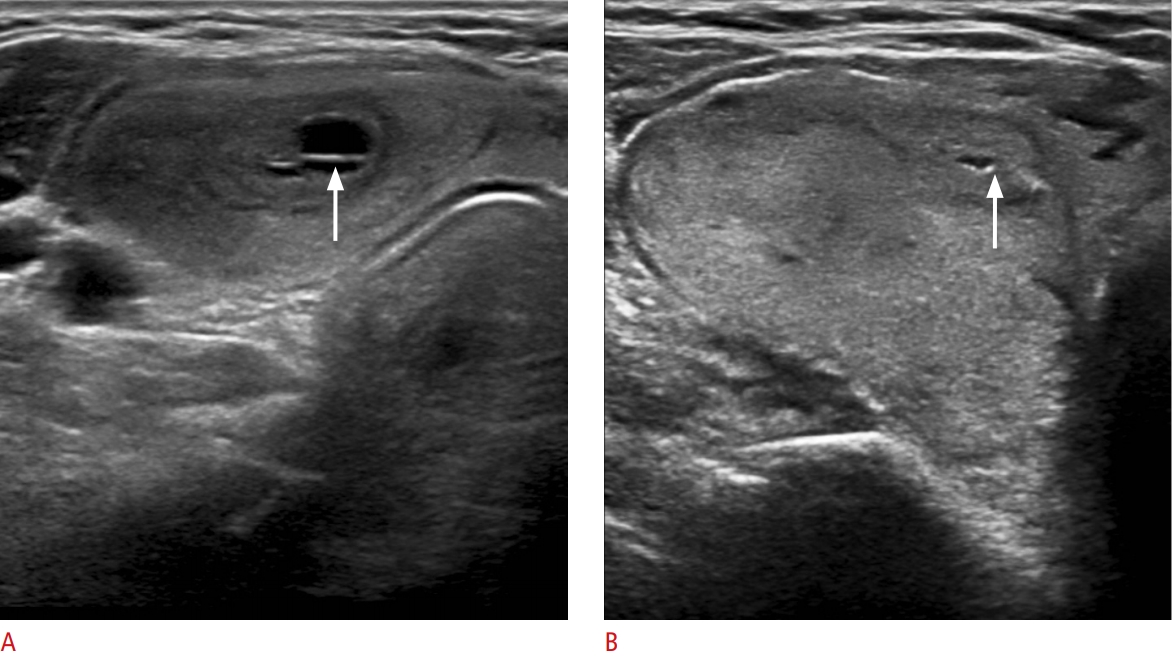 Fig.┬Ā5.Intracystic nonshadowing echogenic foci in a partially cystic nodule of minimally invasive follicular thyroid cancer.Transverse ultrasonography shows a predominantly cystic nodule with numerous intracystic punctate echogenic foci without comet tail artifacts and with triangular comet tail artifacts (tail size, 1.4 mm, 1.1 mm) (arrows).
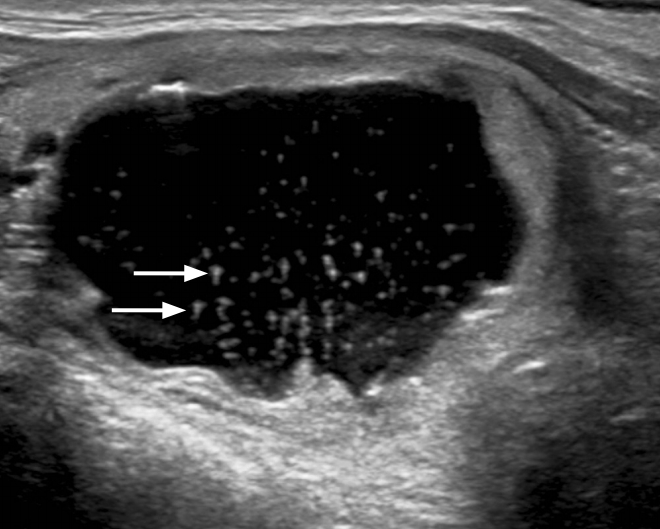 Fig.┬Ā6.Table┬Ā1.Association of nonshadowing echogenic foci with malignancy according to the presence of a comet tail artifact, location, and size Table┬Ā2.Associations with malignancy and malignancy risk of nonshadowing echogenic foci in overall nodules Table┬Ā3.Associations with malignancy and malignancy risk of nonshadowing echogenic foci according to composition and echogenicity Table┬Ā4.Diagnostic values of intrasolid punctate echogenic foci according to the presence of a comet tail artifact, composition, and echogenicity |