Jeon, Kim, Son, and Lee: Tips for finding magnetic resonance imaging-detected suspicious breast lesions using second-look ultrasonography: a pictorial essay
Abstract
Second-look ultrasonography (US) is a targeted breast US examination that evaluates suspicious lesions detected on magnetic resonance imaging (MRI). It is a useful tool for determining the probability of malignancy and facilitating US-guided biopsy. Lesions detected on MRI and US should be correlated accurately, which is challenging in some cases. This article documents second-look US and MRI findings that are correlated with the pathology, and suggests helpful approaches for correlating between the two modalities.
Keywords: Breast neoplasms; Ultrasound; Magnetic resonance imaging
Introduction
Breast magnetic resonance imaging (MRI) is the most sensitive modality for detecting and staging breast cancer [ 1, 2]. However, its specificity is limited, and it is difficult to manage when there are multiple suspicious lesions [ 3]. Additional MRI-detected suspicious breast lesions in patients with breast cancer have a higher probability of malignancy than those in patients without breast cancer [ 4]. Second-look or targeted ultrasonography (US) helps to evaluate suspicious lesions that have been detected on MRI. It is a useful diagnostic tool for determining the probability of malignancy, and it also facilitates biopsy or pre-surgical wire-localization [ 5]. However, an accurate lesion diagnosis requires correlation between MRI and US findings [ 6]. The surgical plan may be changed according to the pathologic results of an additional MRI-detected lesion [ 4]. This article aimed to document second-look US and MRI findings that are correlated with the pathology and to suggest helpful approaches for correlating between the two modalities.
The Clinical Needs and Limitations of Second-Look US
Breast MRI determines the local tumor extent, multifocality, multicentricity, or bilaterality of breast cancer with higher accuracy than mammography or US [ 1]. MRI depicts additional malignant lesions that are not discerned by other imaging techniques in up to 37% of patients [ 2]. Since international recommendations for breast MRI have been published, preoperative staging with MRI has been widely used in patients with breast cancer [ 7]. Because of the increasing application of MRI in clinical practice, the detection of additional suspicious lesions is also increasing [ 7]. This has been accompanied by a greater need for histologic confirmation preoperatively [ 8]. However, MRI-guided biopsy is time-consuming, expensive, and requires special equipment. In this context, second-look US is a cost-effective tool to evaluate suspicious MRI-detected lesions that facilitates US-guided biopsy and pre-surgical wire-localization [ 5, 6]. The detection rate of additional suspicious lesions with second-look US varies from 23% to 89% [ 6, 7]. This high variability can be attributed to the lesion type (mass vs. non-mass) and final lesion diagnosis (malignant vs. benign) [ 7]. Previous studies have reported that US correlation for MRI-detected lesions was more likely for masses than for non-mass enhancement [ 7- 9], because additional MRI-detected lesions are often small and sonographically subtle, especially in cases of non-mass enhancement [ 10]. Malignant lesions are more likely to be identified on second-look US than benign lesions [ 7, 9, 10]. However, a negative second-look US does not exclude malignancy [ 7]. Previous studies reported that 10%-20% of additional MRI-detected lesions that were not visible on US were malignant [ 7]. In cases where suspicious lesions are not visible on US, MRI-guided biopsy should be considered. When US-guided biopsy is performed, the concordance between the imaging findings and pathologic results should be reviewed. MRI-guided biopsy or surgical excision should be considered if a discordant result is obtained. A follow-up examination is required even if the results are concordant benign, because approximately 14% of lesions that underwent US-guided biopsy did not correspond to an MRI-detected lesion, and 29% of them were diagnosed as malignant at re-biopsy [ 4, 7]. In addition, the operatorŌĆÖs experience and differences in technical equipment can impact the detection rate of second-look US. It is important to improve the accuracy of second-look US through careful scanning and correlation.
Tips to Find Additional MRI-Detected Suspicious Lesions Using Second-Look US
Location of the Lesion
To localize and identify the MRI-detected suspicious lesion via second-look US, a clockwise direction is preferable, and the distance from the nipple should be considered ( Fig. 1) [ 11]. Breast MRI is commonly performed with the patient in the prone position, whereas the breast US is performed with the patient in the supine or supine oblique position. In the supine position, all tissue layers are flattened, especially fatty tissue, which is more compressible than other breast structures. In the prone position, the breasts are pendant and less compressed [ 4, 6]. When performing second-look US, it is important to understand that spatial displacement of the lesion may occur due to body positioning differences [ 12, 13]. This frequently occurs in breasts with a greater amount of fatty tissue, and this could cause considerable variability in the apparent lesion position [ 12]. Thus, the scanning range of second-look US should be extended to the quadrants, where MRI lesions are expected to move [ 12, 13]. A lesion located in the subareolar area may be missed due to the nipple shadow. These lesions can be visualized better by pushing the nipple upward with the US probe to remove or reduce posterior shadowing due to the nipple ( Fig. 2) [ 4, 14].
Depth of the Lesion
When correlating MRI and US findings, the lesion depth with respect to the mammary parenchymal zone and its proximity to the anterior and posterior mammary fascia should be considered [ 12, 15]. On US, the mammary fascia appears as thin echogenic lines, encompassing the mammary parenchymal zone. These serve as landmarks to evaluate lesion depth relative to the breast tissue [ 16]. Based on MRI, the lesion should be localized according to the premammary, mammary, and retromammary zones [ 16]. Although the lesion depth is not identical between the two modalities, the corresponding mammary zone remains constant [ 4]. It is possible to predict the depth of the lesion when only the glandular tissue is considered ( Fig. 3). All breast tissue layers, especially fatty tissue, are flattened on US. As the retromammary fat is markedly thinned on US, lesions that abut the posterior mammary fascia are more posteriorly located on US than on MRI ( Fig. 4).
Size and Shape of the Lesion
The size and shape of the lesion can be used to detect the target lesion on US. However, the size and shape of the lesion are not always identical between US and MRI, especially for non-mass enhancements [ 17]. The lesions appear smaller and flatter on US because of the supine positioning of the patient and vertical compression by the US probe ( Fig. 5). Associated findings of the lesion, such as ductal extension, are also helpful for correlation between the two modalities [ 4].
Landmarks
Anatomical breast structures (e.g., vessels, fat lobule distribution, CooperŌĆÖs ligament) near the targeted lesion are viable landmarks to detect the lesion on US and correlate between the two modalities. Izumori et al. [ 14] reported a high identification rate (99%) using anatomical structures as indicators. Specific features of the morphology of the surrounding tissue will help correlate the two modalities ( Fig. 6). Coexisting lesions (e.g., known breast cancer, known fibroadenoma, cyst, scar, implant) are also helpful identifiers for the lesion [ 4, 6]. These landmarks should be carefully reviewed on T2-weighted and pre-contrast T1-weighted images, because anatomical structures are well visualized on these images. It is noteworthy that the distance between two lesions varies between the two modalities according to the amount of fatty tissue in the breast ( Fig. 7).
Complementary US Techniques
Several complementary techniques have been used to detect target lesions on second-look US. Color or power Doppler imaging helps identify the internal vascularity of a solid mass. Malignant lesions have increased blood flow on Doppler imaging, which suggests neoangiogenesis. This finding is consistent with the mechanism of contrast enhancement on MRI ( Fig. 8). Elastography is a complementary technique that reflects the hardness of the lesion. Malignant lesions are typically harder than the surrounding tissue. Elastography is useful for distinguishing true lesions when the US findings are subtle ( Fig. 9) [ 16]. Tissue harmonic imaging improves image contrast and lateral resolution by providing a clearer definition of the lesion margins. Therefore, tissue harmonic imaging identifies subtle isoechoic lesions by revealing hypoechoic lesions to differentiate them from the surrounding fatty tissue ( Fig. 10) [ 18].
Conclusion
When performing second-look US, it is essential to understand the differences between US and MRI in terms of basic principles and breast position. To accurately identify MRI-detected suspicious lesions on second-look US, the lesion location, depth, and size/shape must be considered. Anatomical structures and coexisting lesions can be useful indicators, and complementary US techniques are helpful for identifying subtle breast lesions. Detecting lesions and correlating the findings between MRI and US are important to prevent unnecessary biopsies or delayed diagnoses.
Notes
Author Contributions
Conceptualization: Lee SE, Kim YS. Data acquisition: Jeon T, Lee SE, Kim YS, Son HM. Data analysis or interpretation: Jeon T, Son HM. Drafting of the manuscript: Jeon T. Critical revision of the manuscript: Lee SE, Kim YS, Son HM. Approval of the final version of the manuscript: all authors.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This study was supported by a research grant from Yeungnam University.
References
1. Morrow M, Waters J, Morris E. MRI for breast cancer screening, diagnosis, and treatment. Lancet 2011;378:1804ŌĆō1811.   3. Peters NH, Borel Rinkes IH, Zuithoff NP, Mali WP, Moons KG, Peeters PH. Meta-analysis of MR imaging in the diagnosis of breast lesions. Radiology 2008;246:116ŌĆō124.   4. Park VY, Kim MJ, Kim EK, Moon HJ. Second-look US: how to find breast lesions with a suspicious MR imaging appearance. Radiographics 2013;33:1361ŌĆō1375.   7. Spick C, Baltzer PA. Diagnostic utility of second-look US for breast lesions identified at MR imaging: systematic review and meta-analysis. Radiology 2014;273:401ŌĆō409.   8. Candelaria R, Fornage BD. Second-look US examination of MR-detected breast lesions. J Clin Ultrasound 2011;39:115ŌĆō121.   9. Demartini WB, Eby PR, Peacock S, Lehman CD. Utility of targeted sonography for breast lesions that were suspicious on MRI. AJR Am J Roentgenol 2009;192:1128ŌĆō1134.   10. Meissnitzer M, Dershaw DD, Lee CH, Morris EA. Targeted ultrasound of the breast in women with abnormal MRI findings for whom biopsy has been recommended. AJR Am J Roentgenol 2009;193:1025ŌĆō1029.   11. Carbonaro LA, Tannaphai P, Trimboli RM, Verardi N, Fedeli MP, Sardanelli F. Contrast enhanced breast MRI: spatial displacement from prone to supine patient's position. Preliminary results. Eur J Radiol 2012;81:e771ŌĆōe774.   16. Berg WA, Leung J. Diagnostic imaging: breast. Philadelphia, PA: Elsevier, 2019.
18. Tranquart F, Grenier N, Eder V, Pourcelot L. Clinical use of ultrasound tissue harmonic imaging. Ultrasound Med Biol 1999;25:889ŌĆō894.  
Lesion location with distance from the nipple and the lesion depth with respect to the mammary fascia.
A 46-year-old woman presented with breast cancer in the right upper outer breast. A. A post-contrast-enhanced T1-weighted magnetic resonance (MR) image shows an additional small, irregular enhancing mass (arrows) in the 6-oŌĆÖclock position of her right breast, 2 cm from the nipple, abutting the anterior mammary fascia (white dashed line). B, C. Second-look ultrasonography (US) detects an ill-defined isoechoic lesion (arrows) at the corresponding location. A lesion that abuts the anterior mammary fascia on MR imaging will also abut the corresponding anterior mammary fascia (black dashed line) on US. Color Doppler imaging shows the vascularity of the lesion (arrows). Excision with US-guided needle localization confirmed ductal carcinoma in situ.
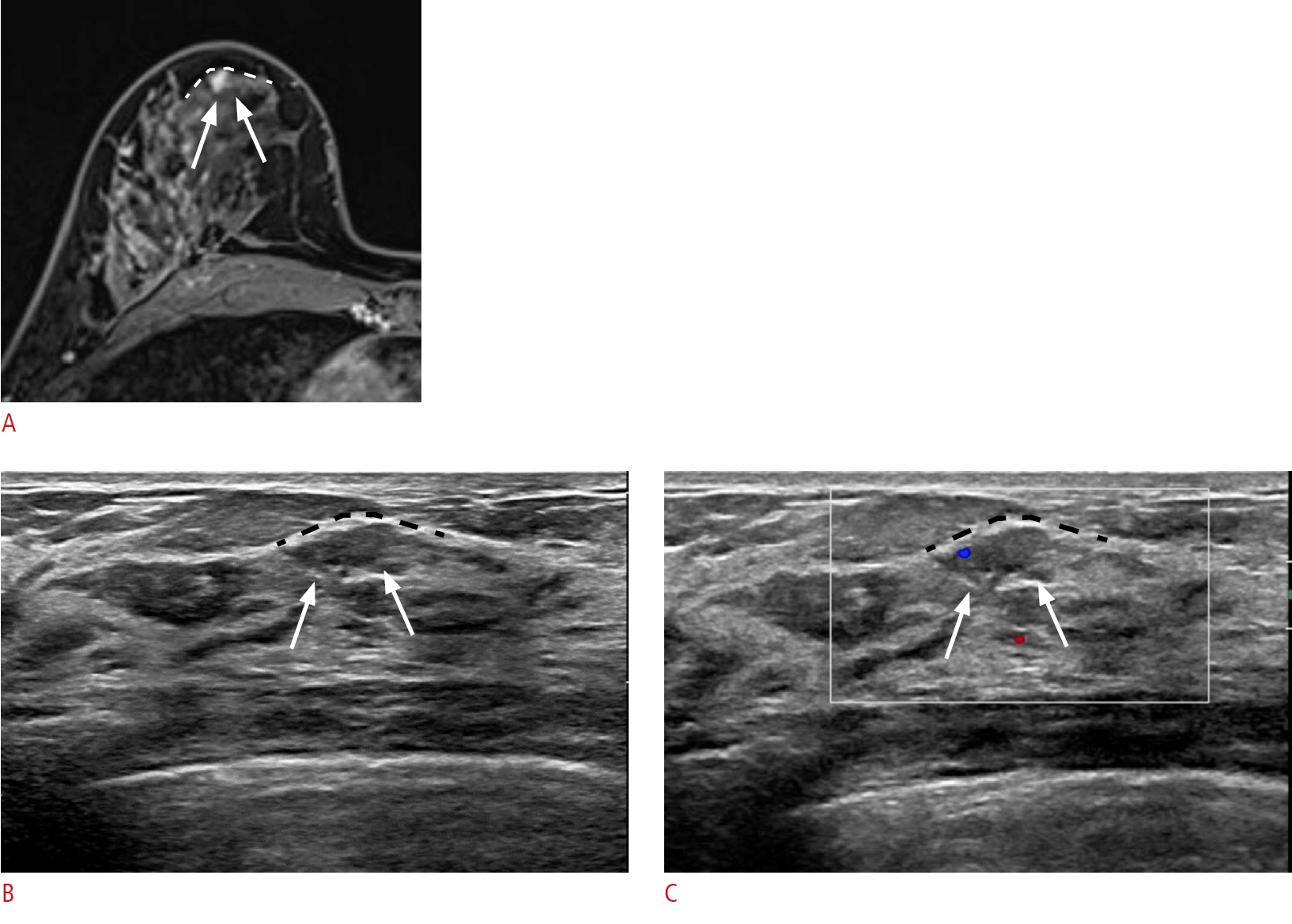 Fig.┬Ā1.
A lesion in the subareolar area.
A 66-year-old woman presented with breast cancer in the upper outer quadrant of the right breast. A. Dynamic contrast-enhanced and subtraction T1-weighted axial magnetic resonance image shows the breast cancer (arrowhead) in the right upper quadrant of the right breast and another small enhancing mass (arrow) in the subareolar area. B. Second-look ultrasonography (US) detects a corresponding hypoechoic mass (arrow) beneath the nipple. Excision with US-guided needle localization confirmed sclerosing adenosis. N, nipple.
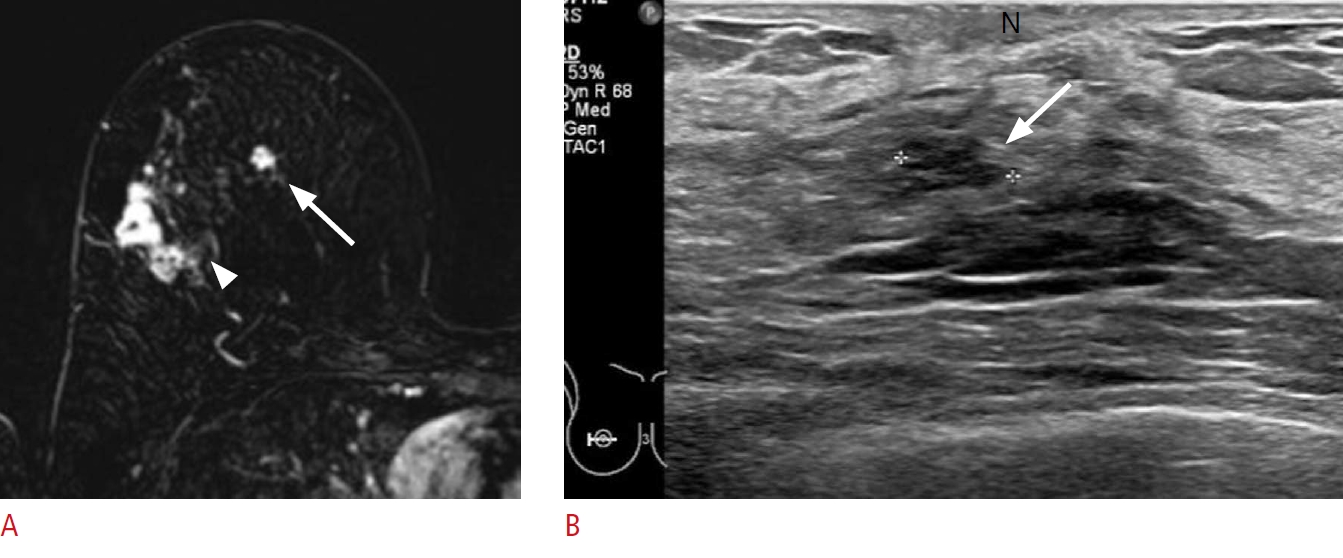 Fig.┬Ā2.
Lesion depth within the breast glandular tissue.
A 59-year-old woman presented with breast cancer in the upper outer quadrant of the left breast. A. A post-contrast-enhanced T1-weighted magnetic resonance image shows an additional small enhancing mass (white arrow) at the 11-oŌĆÖclock position of the left breast, 6 cm from the nipple. The mass is located in the middle depth within the glandular tissue (white dashed lines). B, C. A corresponding ill-defined hypoechoic mass (black arrow) is detected on second-look ultrasonography (US), considering the lesion depth within the glandular tissue (black dashed lines). Blood vessels (arrowheads on A and C) located on the superficial side of the target lesion serve as landmarks to detect the lesion. Excision with US-guided needle localization confirmed an invasive carcinoma.
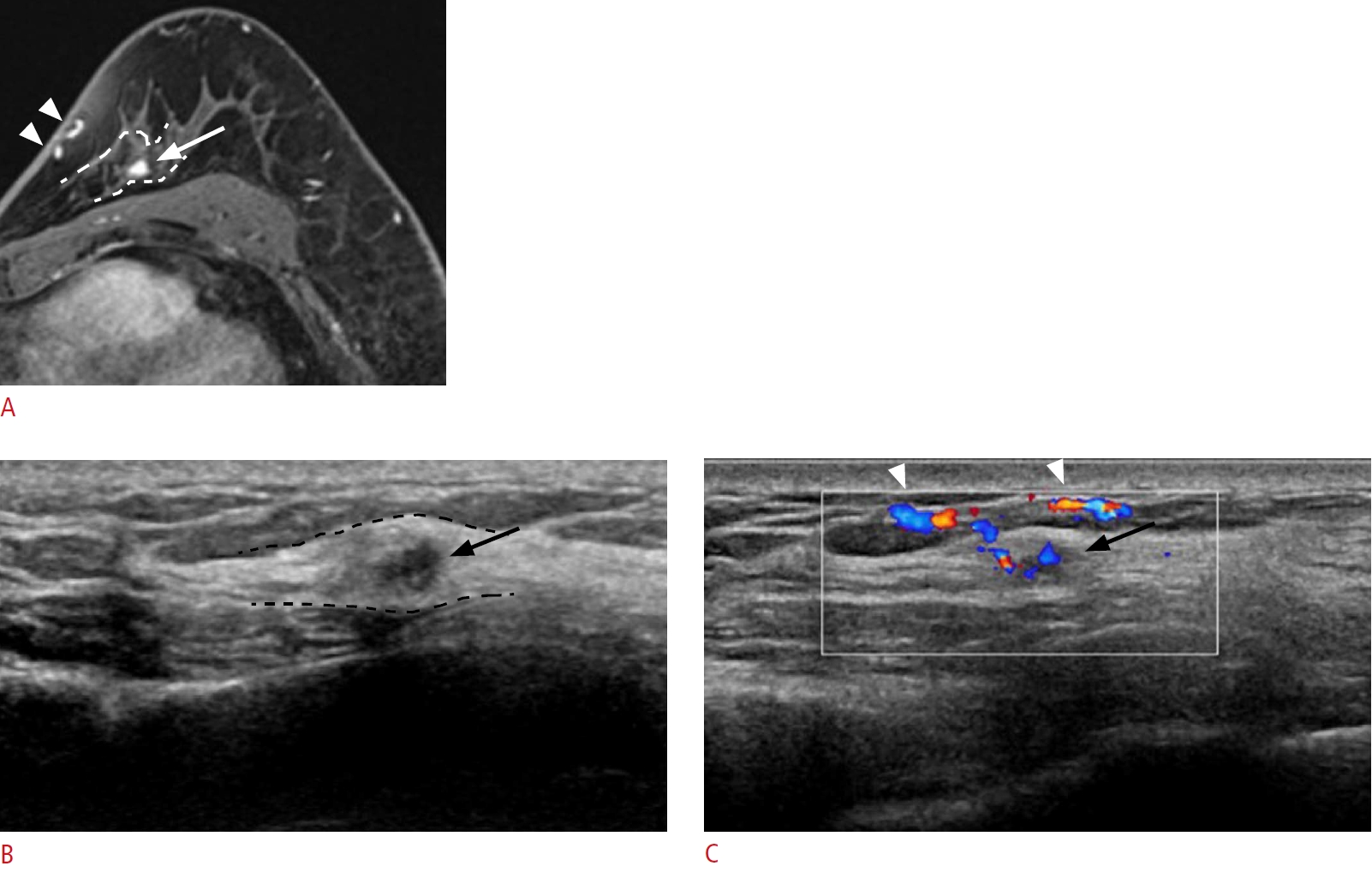 Fig.┬Ā3.
Lesion depth within the surrounding tissue.
A 57-year-old woman presented with right breast cancer. A. A post-contrast-enhanced T1-weighted magnetic resonance image shows an additional focal linear non-mass enhancement (white arrow) in the 2-oŌĆÖclock position of her left breast, 3.5 cm from the nipple, abutting the posterior mammary fascia. B, C. Second-look ultrasonography (US) detects a corresponding ill-defined linear hypoechoic non-mass lesion with architectural distortion (white arrow). The vascularity of the lesion is appreciated in color Doppler imaging. The retromammary fat (yellow double-headed arrows on A and B) is markedly compressed on US, and the lesion is more posteriorly located on US than on magnetic resonance imaging. Excision with US-guided needle localization confirmed an invasive carcinoma.
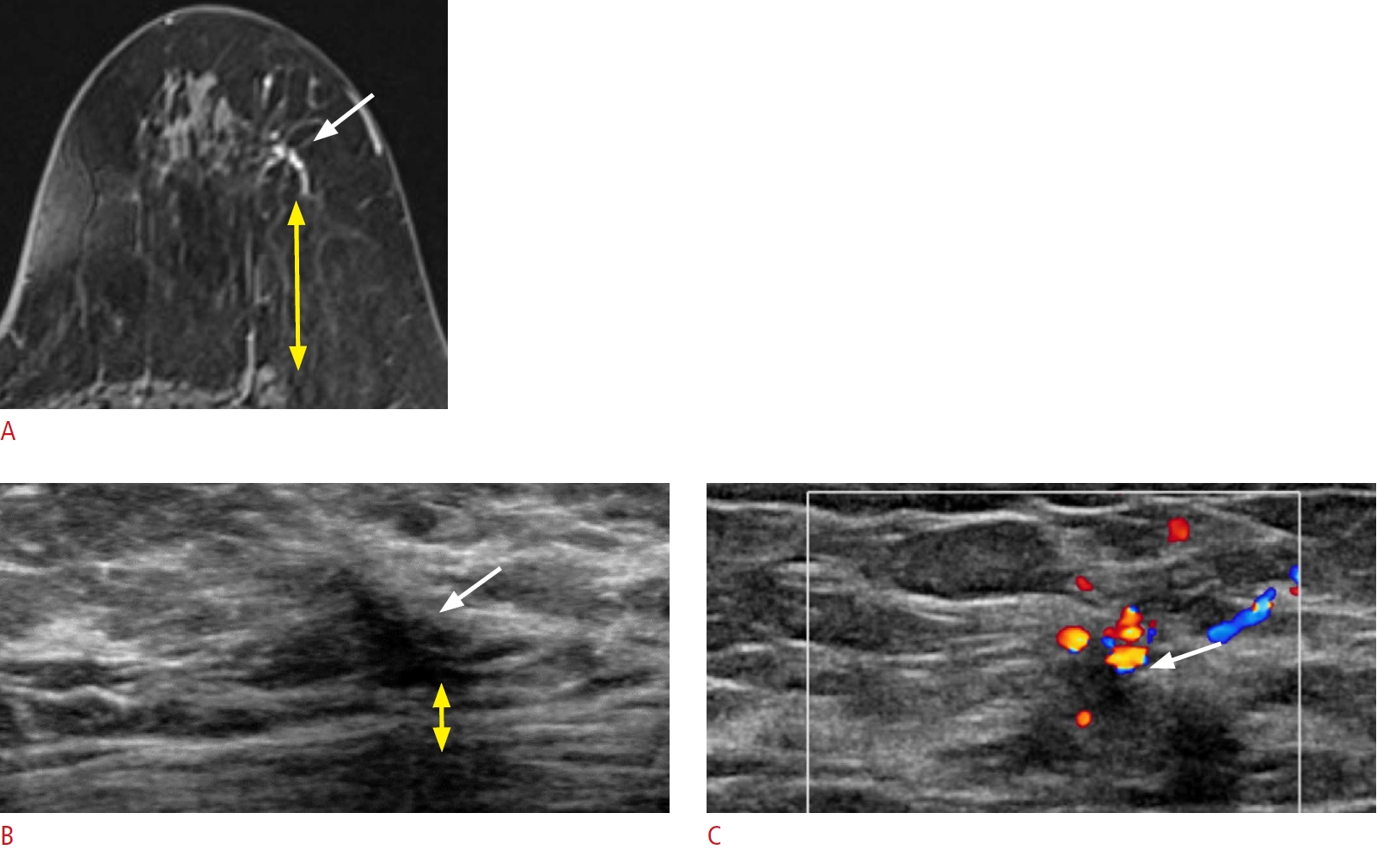 Fig.┬Ā4.
Lesion detection with its size and shape.
A 41-year-old woman presented with left breast cancer. A, B. Post-contrast-enhanced T1-weighted axial magnetic resonance images show an additional irregular enhancing mass (arrow) in the 9-oŌĆÖclock position of her right breast, 6 cm from the nipple. C-E. A corresponding ill-defined isoechoic lesion (arrow) is detected on second-look ultrasonography (US). The lesion appears flat on US, and the vascularity is appreciated on color Doppler imaging. Excision with US-guided needle localization confirmed pseudoangiomatous stromal hyperplasia.
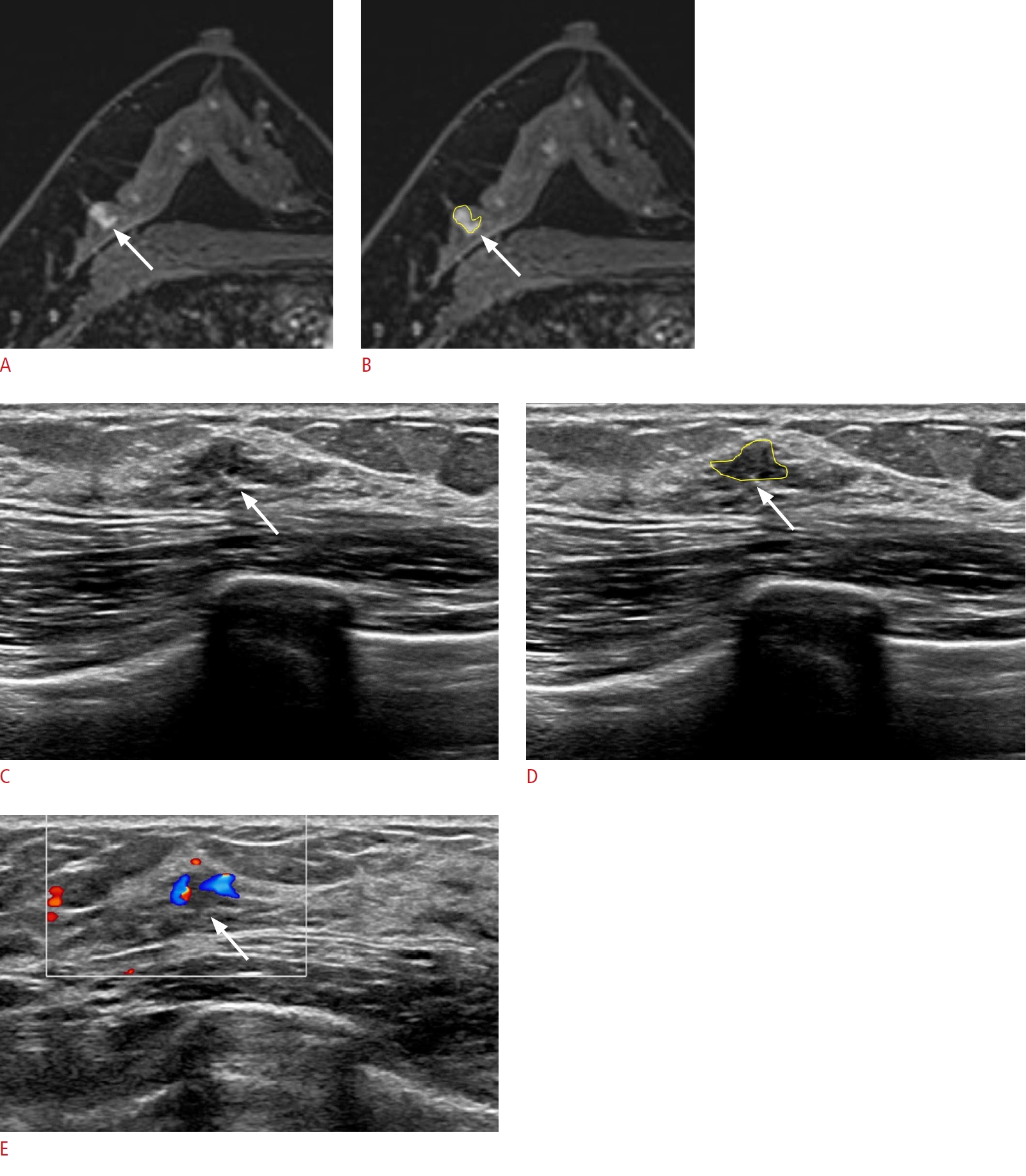 Fig.┬Ā5.
Lesion detection with the surrounding tissue morphology.
A 45-year-old woman presented with left breast cancer. A, B. Post-contrast-enhanced T1-weighted magnetic resonance (MR) images show an additional enhancing mass (arrow) at the 11-oŌĆÖclock position of her right breast, 5 cm from the nipple. C. Second-look ultrasonography (US) detects a corresponding ill-defined isoechoic lesion (arrow). The surrounding fibroglandular tissue exhibits a hook appearance (yellow lines on B and C) on both breast MR imaging and US. Excision with US-guided needle localization confirmed atypical ductal hyperplasia.
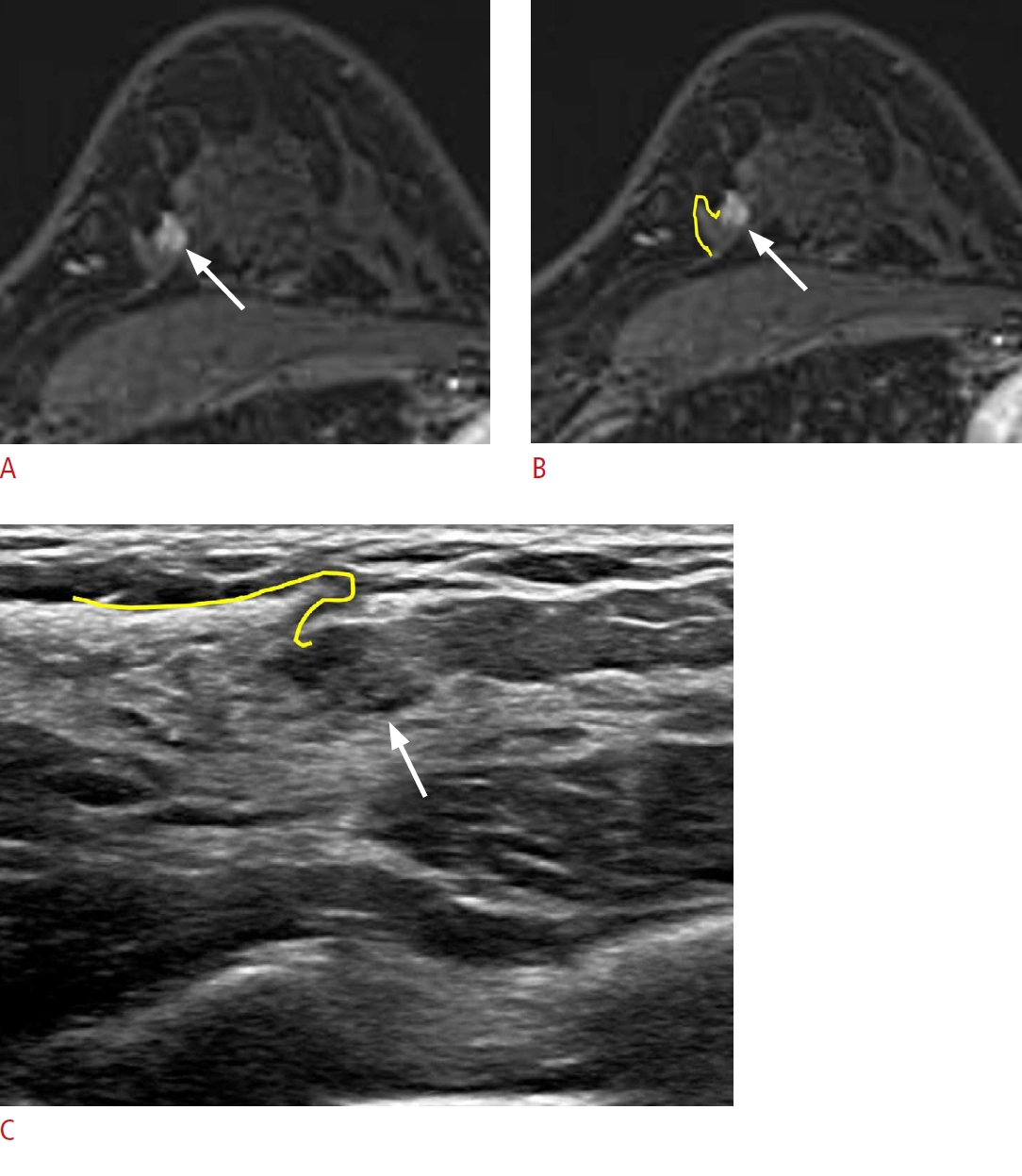 Fig.┬Ā6.
Lesion detection with a coexisting lesion as a landmark.
A 59-year-old woman presented with right breast cancer. A. A post-contrast-enhanced T1-weighted magnetic resonance (MR) image shows an additional small enhancing mass (arrow), 1 cm from the main mass (arrowhead). B. Second-look ultrasonography (US) detects a corresponding small, irregular hypoechoic mass (arrow), 2 cm from the main mass (arrowhead). Excision confirmed an invasive carcinoma. The malignant mass serves as a landmark for detecting the additional lesion. However, the distance between the two lesions (yellow double-headed arrows on A and B) differs between MR imaging and US.
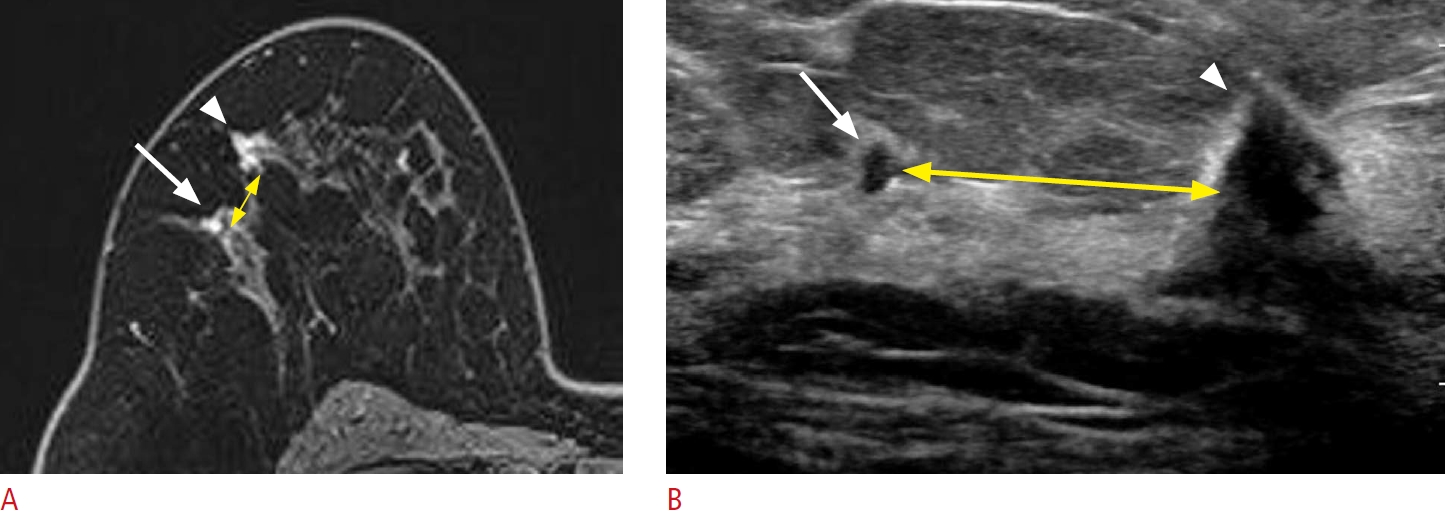 Fig.┬Ā7.
Lesion detection with color Doppler imaging.
A 39-year-old woman presented with breast cancer in the upper inner quadrant of the left breast. A. A post-contrast-enhanced T1-weighted magnetic resonance image shows an oval enhancing mass (arrow) in the 2-oŌĆÖclock peri-areolar area. B-D. Second-look ultrasonography (US) detects a small oval isoechoic mass (arrow) at the corresponding location. The mass exhibits significantly increased vascularity on color Doppler imaging. Excision with US-guided needle localization confirmed a ductal carcinoma in situ. N, nipple.
 Fig.┬Ā8.
Lesion detection with elastography.
A 70-year-old woman presented with breast cancer in the 3-oŌĆÖclock region of the left breast. A. Post-contrast T1-weighted maximum intensity projection shows known breast cancer (arrowhead) in the left 3-oŌĆÖclock region and additional non-mass enhancement (arrow) in the left subareolar area. B. Second-look ultrasonography (US) detects a corresponding ill-defined isoechoic non-mass lesion (arrow) in the left subareolar area, 1 cm from the known breast cancer (arrowhead). C. Elastography demonstrates hardness (blue, hard; red, soft), which helped distinguish the lesion (arrow) from the adjacent normal breast and fatty tissues. Excision with US-guided needle localization confirmed atypical
ductal hyperplasia.
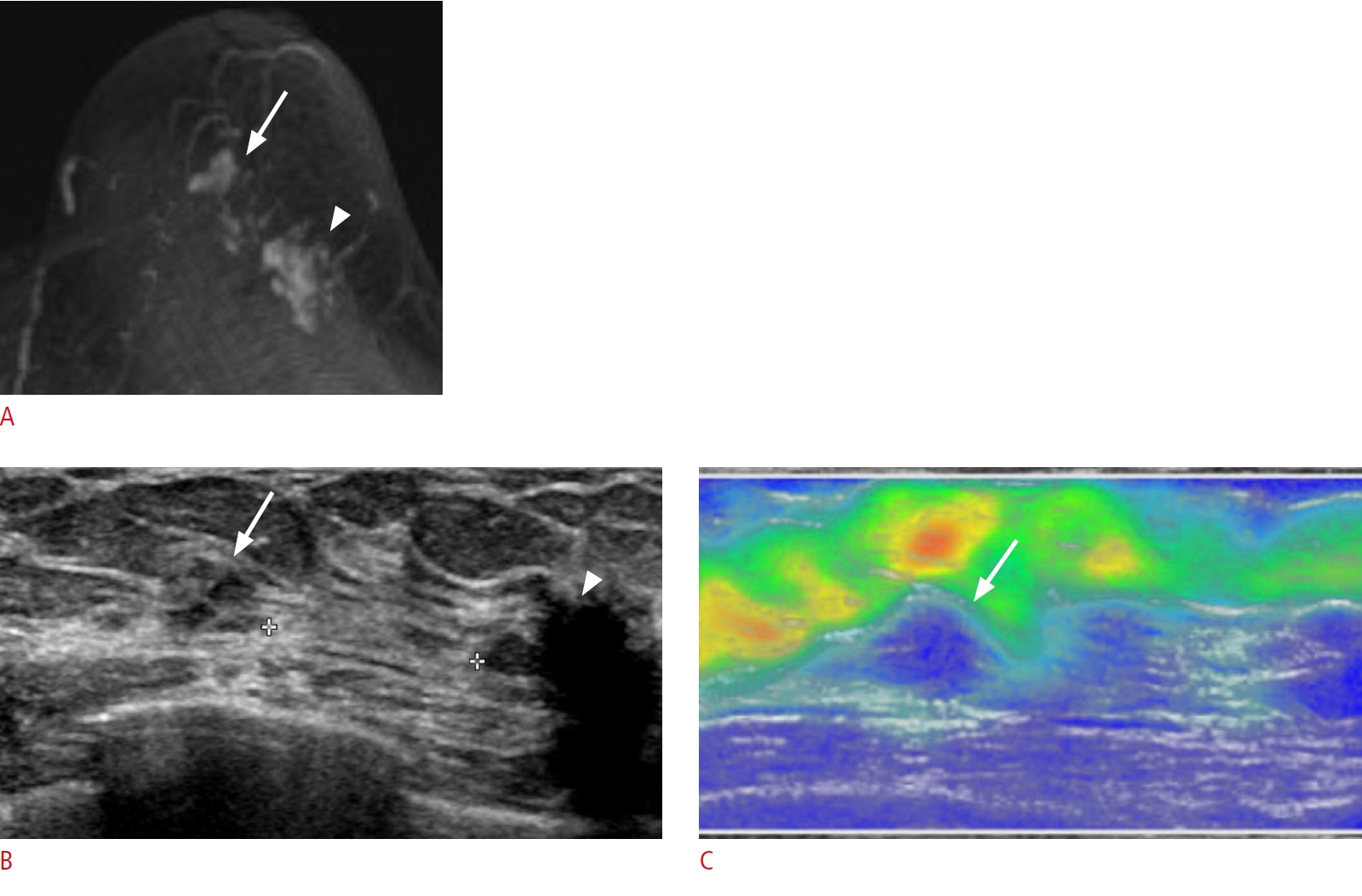 Fig.┬Ā9.
Lesion detection with tissue harmonic imaging.
A 46-year-old woman presented with breast cancer in the lower central portion of her left breast. A. A post-contrast-enhanced T1-weighted magnetic resonance image shows two oval, enhancing masses at the 1-oŌĆÖclock region of the left breast, 8 cm from the nipple (arrows). B. B-mode ultrasonography (US) detects two corresponding ill-defined isoechoic masses (arrows). C. Tissue harmonic imaging accentuates the isoechoic masses (arrows), helping to differentiate the isoechoic masses from the adjacent fatty tissue. Excision with US-guided needle localization confirmed invasive carcinomas.
 Fig.┬Ā10.
|
|
| TOOLS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
METRICS

|
|
|
|
|
|



 Print
Print facebook
facebook twitter
twitter Linkedin
Linkedin google+
google+










 Download Citation
Download Citation PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC




