AbstractPurposeThis study was conducted to assess the role of renal Doppler ultrasonography (US) in predicting non-diabetic kidney disease (NDKD) in patients with diabetes, using histologic findings as the reference standard.
MethodsFifty-nine consecutive patients with diabetes who underwent renal Doppler US and native kidney biopsy were included in this retrospective, single-institutional study. Based on histologic findings, patients were classified as having diabetic nephropathy (DN) or NDKD. Renal Doppler US findings, including cortical echogenicity, corticomedullary differentiation, and the resistive index (RI), were compared between DN and NDKD. A subgroup analysis according to chronic kidney disease (CKD) status was also performed.
ResultsCortical echogenicity and corticomedullary differentiation showed no significant differences between DN and NDKD (P=0.887 and P>0.99, respectively), whereas the RI was significantly higher in patients with DN than in those with NDKD (P=0.032). The subgroup analysis revealed a significant difference in the RI between DN and NDKD in patients with diabetes and CKD (P=0.010), but a significant difference was not found in those without CKD (P=0.713). When limited to patients with diabetes and CKD, the RI had an area under the curve value of 0.759, sensitivity of 57.1%, specificity of 81.0%, positive likelihood ratio of 3.0, and negative LR of 0.5 for predicting NDKD, using a cutoff value of ≤0.69.
Renal involvement secondary to diabetes mellitus (DM) is the most common cause of chronic kidney disease (CKD) worldwide [1]. Diabetic nephropathy (DN) is typically diagnosed based on clinical factors, including the duration of diabetes, the presence of neuropathy, retinopathy, or other complications, as well as the development of gradual and progressive proteinuria [2]. However, non-diabetic kidney disease (NDKD) may also occur in some patients with diabetes. According to a recent meta-analysis, the prevalence of NDKD ranges from 6.5% to 94% [3].
In patients with diabetes, it is important to distinguish NDKD from DN because renal function could be restored through treatment in some cases of NDKD [4-6]. Previous investigators have shown that renal Doppler ultrasonography (US) effectively reflects the progression of DN [7-11]. However, these studies relied on clinical diagnoses of DN and did not compare it with NDKD, which can also occur in patients with diabetes. To the best of the authors’ knowledge, the role of renal Doppler US in predicting NDKD in patients with diabetes remains unclear. Therefore, this study aimed to evaluate the utility of renal Doppler US in predicting NDKD in patients with diabetes by comparing renal Doppler US findings between biopsy-based DN and biopsy-based NDKD.
The institutional review board of SMG-SNU Boramae Medical Center (IBR No. 20-2023-17) approved the data collection for this retrospective study, and informed consent was waived. The waiver of informed consent was granted based on the retrospective nature of the study and other ethical considerations, as approved by the institutional review board.
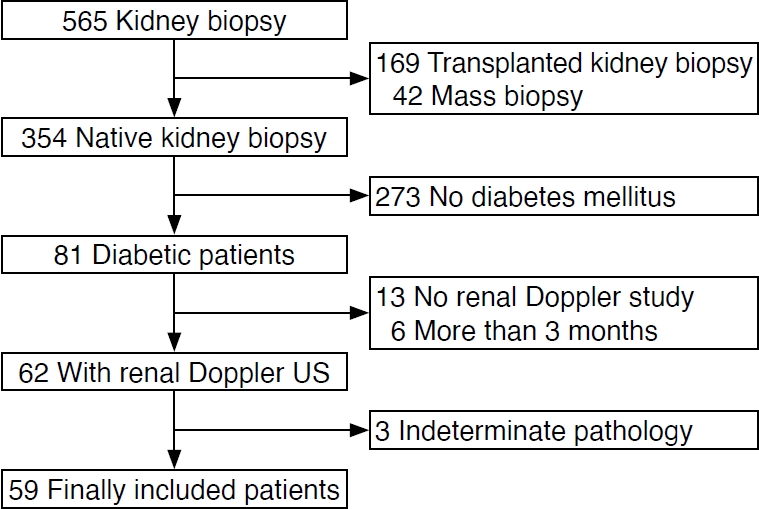
A retrospective single-center cohort of consecutive patients with diabetes who underwent kidney biopsy between July 2017 and December 2021 was analyzed]. Eighty-one patients who were diagnosed with DM [12] underwent native kidney biopsy due to atypical clinical features. Of these, 19 patients were excluded because they did not have a renal Doppler US study before the biopsy (n=13) or had a renal Doppler US study performed more than 3 months prior to biopsy (n=6). Three additional patients were excluded due to indeterminate biopsy results. Finally, a total of 59 patients with diabetes (44 men, 15 women; mean age, 62 years; age range, 30 to 83 years) were included in the analysis (Fig. 1).
US examinations were performed by three genitourinary radiologists (C.K.S., with 21 years of experience; M.H.M., with 18 years of experience; M.S.L., with 7 years of experience) using a Philips IU22 ultrasound system (Philips Healthcare, Bothell, WA, USA) equipped with a curvilinear C5-1 broadband transducer or an Aplio 500 system (Toshiba Medical Systems Corp., Otawara, Japan) equipped with a 6C1 curvilinear transducer. A standard gray-scale examination and multiple Doppler waveforms of the kidney were obtained and transferred to the picture archiving and communication system (Marosis M-view, Infinitt, Seoul, Korea). Color Doppler US was used to identify intrarenal arteries, and Doppler signals were obtained from the interlobar arteries along the border of medullary pyramids [11]. To minimize error in the measurement of the resistive index (RI), Doppler waveforms were maximized using the lowest pulse repetition possible without aliasing. The RI was automatically calculated at the time of ultrasound examinations using the following formula: RI=PSV-EDV/PSV, where PSV represents peak systolic velocity, and EDV denotes end-diastolic velocity. The ultrasound examinations were completed in 15 minutes or less.
Renal cortical echogenicity and corticomedullary differentiation were retrospectively determined by consensus agreement of two reviewers (M.H.M., with 18 years of experience; S.I.J., with 17 years of experience), who were blinded to the renal function and the pathologic results of study population. Renal cortical echogenicity was categorized as normal (hypoechoic to the adjacent liver parenchyma), equal (isoechoic to the adjacent liver parenchyma), or increased (hyperechoic to the adjacent liver parenchyma) [13,14]. If the liver parenchyma's echogenicity appeared increased due to fatty liver, spleen echogenicity was used for comparison. Corticomedullary differentiation was subjectively categorized as either normal or accentuated [15,16]. The acquisition of Doppler waveforms for RI measurement varied from 1 to 6 per patient, with a median value of 3. RI values were collected retrospectively, and the mean RI values were calculated for each patient for subsequent analysis.
Histologic diagnoses were prospectively provided by a pathologist (J.H.P.) specialized in renal pathology as part of their daily clinical practice. Based on the histologic results, patients were allocated to three categories: (1) DN, (2) NDKD, and (3) NDKD superimposed on DN. Categories 2 and 3 were grouped together into the NDKD group because the presence of NDKD may affect treatment plans in patients with diabetes in whom kidney disease is suspected.
Differences between the DN and NDKD groups were assessed using the Mann-Whitney test (MedCalc, version 18.6, MedCalc Software, Ostend, Belgium) for continuous variables and Fisher's exact test (R, version 4.4.1, The R Foundation, Vienna, Austria) for categorical variables. Since renal function in patients with diabetes may influence RI [11], a subgroup analysis for RI was also conducted based on the presence or absence of CKD. CKD was defined as a glomerular filtration rate below 60 mL/min per 1.73 m2 for a duration of at least 3 months [17]. Receiver operating characteristic analysis was employed to evaluate the diagnostic performance of Doppler US in diagnosing NDKD in patients with diabetes, and the cutoff values were determined using the highest Youden index (MedCalc, version 18.6). A P-value below 0.05 was considered to indicate a statistically significant difference.
Of 59 patients with diabetes, 29 (49.2%) had a pathologic diagnosis of DN, 20 (33.9%) had NDKD, and 10 (16.9%) had NDKD superimposed on DN. Nephropathy in the NDKD group (n=30) is summarized in Table 1. Acute tubulointerstitial nephritis was the most common nephropathy in the NDKD group (26.7%), followed by minimal change disease (13.3%), IgA nephropathy (13.3%), and membranoproliferative glomerulonephritis (13.3%). CKD was found in 72.4% (21/29) of patients with DN and 46.7% (14/30) of patients in the NDKD group (P=0.064).
The renal length in the DN group ranged from 8.5 cm to 14 cm, with a median length of 10.7 cm, while that in the NDKD group ranged from 7.9 cm to 13.8 cm, with a median value of 10.8 cm (P=0.930). Table 2 summarizes the Doppler US findings of the study population. Parenchymal echogenicity and corticomedullary differentiation showed no significant differences between the DN and NDKD groups (P=0.887 and P>0.99, respectively). However, the RI of Doppler spectra was significantly different between the DN and NDKD groups (P=0.032). In the subgroup analysis, patients without CKD (n=24) did not show a significant difference in the RI between the DN and NDKD groups (P=0.713), whereas a significant difference in the RI was observed between the DN and NDKD groups (P=0.010) (Fig. 2). In patients with diabetes and CKD, the RI in the DN group (median, 0.74; 95% confidence interval [CI], 0.71 to 0.76) was significantly higher than the RI in the NDKD group (median, 0.69; 95% CI, 0.66 to 0.72). Furthermore, in patients with diabetes and CKD, the RI had an area under the receiver operating characteristic curve (AUC) value of 0.759 (P=0.001), sensitivity of 57.1% (95% CI, 28.9% to 82.3%), specificity of 81.0% (95% CI, 58.1% to 94.6%), positive likelihood ratio (LR) of 3.0 (95% CI, 1.1 to 8.1), and negative LR of 0.5 (95% CI, 0.3 to 1.0) for the diagnosis of NDKD, using a cutoff value of ≤0.69 (Fig. 3).
The intrarenal RI, a reflection of renal vascular resistance, has been utilized in the assessment of kidney diseases. Evaluating intrarenal RI can help pinpoint the location of kidney disease, as active disease in the tubule-interstitial or vascular compartment typically exhibits elevated RI, while disease confined to the glomeruli usually presents with normal RI [18]. Intrarenal RI measurements have also been reported to be useful in differentiating acute tubular necrosis from prerenal acute kidney injury [19,20]. In patients with diabetes, intrarenal RI has been shown to predict the functional status of the kidneys. Elevated RI is uncommon in the early stages without renal dysfunction, but it is frequently observed in later stages with clinical renal dysfunction [7-11]. The mechanism responsible for the increased RI in patients with DM remains unknown, but arteriosclerosis is thought to be the key factor [8,21,22]. Similar to previous research, the present study found that the intrarenal RI was higher in patients with DN who had CKD than in those with DN who did not have CKD. However, unlike previous studies, the intrarenal RI was found to predict renal function in pathologically-proven cases of DN, which represents a notable strength of the present study.
Kidney biopsy is typically performed in patients with diabetes who exhibit atypical clinical and laboratory features. However, the indications for renal biopsy in patients with diabetes remain unclear and are largely based on subjective decisions made by physicians and institutional policies [4,23-25]. Several factors, such as severe proteinuria or rapidly progressing proteinuria, the absence of retinopathy, a short duration of diabetes, the presence of hematuria, and acute deterioration of renal function, have been identified as clinical predictors of NDKD [26,27]. In the present study, RI demonstrated an AUC value of 0.759 (P=0.001), a sensitivity of 57.1% (95% CI, 28.9% to 82.3%), a specificity of 81.0% (95% CI, 58.1% to 94.6%), a positive LR of 3.0 (95% CI, 1.1% to 8.1%), and a negative LR of 0.5 (95% CI, 0.3% to 1.0%) for diagnosing NDKD in patients with diabetes and CKD, using a cutoff value of ≤0.69 (Fig. 3). Based on these findings, it is proposed that intrarenal RI measurement could serve as a criterion for indicating kidney biopsy in patients with diabetes. However, it is important to note that patients with diabetes without CKD will not benefit from intrarenal RI evaluation in predicting NDKD (Table 2). To the best of the authors’ knowledge, this is the first study to employ US as a criterion for predicting NDKD in patients with diabetes
The primary limitation of this study is its retrospective design. In this biopsy-based investigation, kidney biopsies were conducted for diagnostic purposes rather than for research purposes. Although the indications for renal biopsy in patients with diabetes are not well-defined in current clinical practice [23], there may have been selection bias in this study, as the indications for renal biopsy were determined at the discretion of the treating clinicians. Ultrasound examinations were performed by three experienced radiologists who specialize in genitourinary imaging. While these radiologists typically attempted to follow the recommendations of the Korean Society of Ultrasound in Medicine (KSUM), the lack of uniformity in the ultrasound examinations represents another limitation of this retrospective study. Additionally, the small sample size and single-institution nature of the study may limit the generalizability of these findings. The institution where this study was conducted is a tertiary referral center that performs over 100 kidney biopsies annually. However, obtaining a larger sample size was challenging, as kidney biopsies are not routinely performed in patients with diabetes with suspected kidney disease. Moreover, this study was carried out at a single institution. To confirm and generalize the findings, large-scale prospective biopsy-based studies with standardized indications for biopsy are needed.
In conclusion, patients with diabetes who present with signs or symptoms of kidney disease may have NDKD and should be evaluated accordingly. Based on the high prevalence of NDKD in patients with diabetes and CKD who had normal RIs, a normal intrarenal RI may be a potential indicator for predicting NDKD in these patients. The authors hope that these findings will be helpful in managing patients with diabetes and CKD.
NotesAUTHOR CONTRIBUTION Conceptualization: Jung SI, Moon MH. Data acquisition: Moon MH, Sung CK, Lee MS. Data analysis or interpretation: Jung SI, Moon MH, Park JH, Oh S. Drafting of the manuscript: Jung SI, Moon MH, Park JH, Oh S. Critical revision of the manuscript: Moon MH, Sung CK, Lee MS. Approval of the final version of the manuscript: all authors. References1. Ritz E, Rychlik I, Locatelli F, Halimi S. End-stage renal failure in type 2 diabetes: a medical catastrophe of worldwide dimensions. Am J Kidney Dis 1999;34:795–808.
2. Qi C, Mao X, Zhang Z, Wu H. Classification and differential diagnosis of diabetic nephropathy. J Diabetes Res 2017;2017:8637138.
3. Fiorentino M, Bolignano D, Tesar V, Pisano A, Biesen WV, Tripepi G, et al. Renal biopsy in patients with diabetes: a pooled meta-analysis of 48 studies. Nephrol Dial Transplant 2017;32:97–110.
4. Espinel E, Agraz I, Ibernon M, Ramos N, Fort J, Seron D. Renal biopsy in type 2 diabetic patients. J Clin Med 2015;4:998–1009.
5. Liu D, Huang T, Chen N, Xu G, Zhang P, Luo Y, et al. The modern spectrum of biopsy-proven renal disease in Chinese diabetic patients-a retrospective descriptive study. PeerJ 2018;6:e4522.
6. Tan J, Zwi LJ, Collins JF, Marshall MR, Cundy T. Presentation, pathology and prognosis of renal disease in type 2 diabetes. BMJ Open Diabetes Res Care 2017;5:e000412.
7. Soldo D, Brkljacic B, Bozikov V, Drinkovic I, Hauser M. Diabetic nephropathy: comparison of conventional and duplex Doppler ultrasonographic findings. Acta Radiol 1997;38:296–302.
8. Ishimura E, Nishizawa Y, Kawagishi T, Okuno Y, Kogawa K, Fukumoto S, et al. Intrarenal hemodynamic abnormalities in diabetic nephropathy measured by duplex Doppler sonography. Kidney Int 1997;51:1920–1927.
9. Brkljacic B, Mrzljak V, Drinkovic I, Soldo D, Sabljar-Matovinovic M, Hebrang A. Renal vascular resistance in diabetic nephropathy: duplex Doppler US evaluation. Radiology 1994;192:549–554.
10. Platt JF, Rubin JM, Ellis JH. Diabetic nephropathy: evaluation with renal duplex Doppler US. Radiology 1994;190:343–346.
11. Kim SH, Kim SM, Lee HK, Kim S, Lee JS, Han MC. Diabetic nephropathy: duplex Doppler ultrasound findings. Diabetes Res Clin Pract 1992;18:75–81.
12. American Diabetes Association. (2) Classification and diagnosis of diabetes. Diabetes Care 2015;38 Suppl:S8–S16.
13. Huntington DK, Hill SC, Hill MC. Sonographic manifestations of medical renal disease. Semin Ultrasound CT MR 1991;12:290–307.
14. Platt JF, Rubin JM, Bowerman RA, Marn CS. The inability to detect kidney disease on the basis of echogenicity. AJR Am J Roentgenol 1988;151:317–319.
15. Hricak H, Cruz C, Romanski R, Uniewski MH, Levin NW, Madrazo BL, et al. Renal parenchymal disease: sonographic-histologic correlation. Radiology 1982;144:141–147.
16. Quaia E, Bertolotto M. Renal parenchymal diseases: is characterization feasible with ultrasound? Eur Radiol 2002;12:2006–2020.
18. Platt JF, Ellis JH, Rubin JM, DiPietro MA, Sedman AB. Intrarenal arterial Doppler sonography in patients with nonobstructive renal disease: correlation of resistive index with biopsy findings. AJR Am J Roentgenol 1990;154:1223–1227.
19. Platt JF, Rubin JM, Ellis JH. Acute renal failure: possible role of duplex Doppler US in distinction between acute prerenal failure and acute tubular necrosis. Radiology 1991;179:419–423.
20. Izumi M, Sugiura T, Nakamura H, Nagatoya K, Imai E, Hori M. Differential diagnosis of prerenal azotemia from acute tubular necrosis and prediction of recovery by Doppler ultrasound. Am J Kidney Dis 2000;35:713–719.
21. Boeri D, Derchi LE, Martinoli C, Simoni G, Sampietro L, Storace D, et al. Intrarenal arteriosclerosis and impairment of kidney function in NIDDM subjects. Diabetologia 1998;41:121–124.
22. Derchi LE, Martinoli C, Saffioti S, Pontremoli R, De Micheli A, Bordone C. Ultrasonographic imaging and Doppler analysis of renal changes in non-insulin-dependent diabetes mellitus. Acad Radiol 1994;1:100–105.
23. Chemouny JM, Sannier A, Hanouna G, Raimbourg Q, Daugas E, Vigneau C, et al. Criteria to indicate kidney biopsy in type 2 diabetic patients with proteinuria: survey among French nephrologists. Nephrol Ther 2019;15:524–531.
24. Gonzalez Suarez ML, Thomas DB, Barisoni L, Fornoni A. Diabetic nephropathy: is it time yet for routine kidney biopsy? World J Diabetes 2013;4:245–255.
25. Bermejo S, Pascual J, Soler MJ. The current role of renal biopsy in diabetic patients. Minerva Med 2018;109:116–125.
Renal Doppler US of patients with diabetes and CKD.A. A 42-year-old man with biopsy-proven DN shows an increased RI of 0.76. B. A 54-year-old man with biopsy-proven MPGN shows a normal RI of 0.57. C. A 62-year-old man with biopsyproven MCD superimposed on DN shows a normal RI of 0.68. US, ultrasonography; CKD, chronic kidney disease; DN, diabetic nephropathy; RI, resistive index; MPGN, membranoproliferative glomerulonephritis; MCD, minimal change disease; PSV, peak systolic velocity; EDV, end-diastolic velocity.
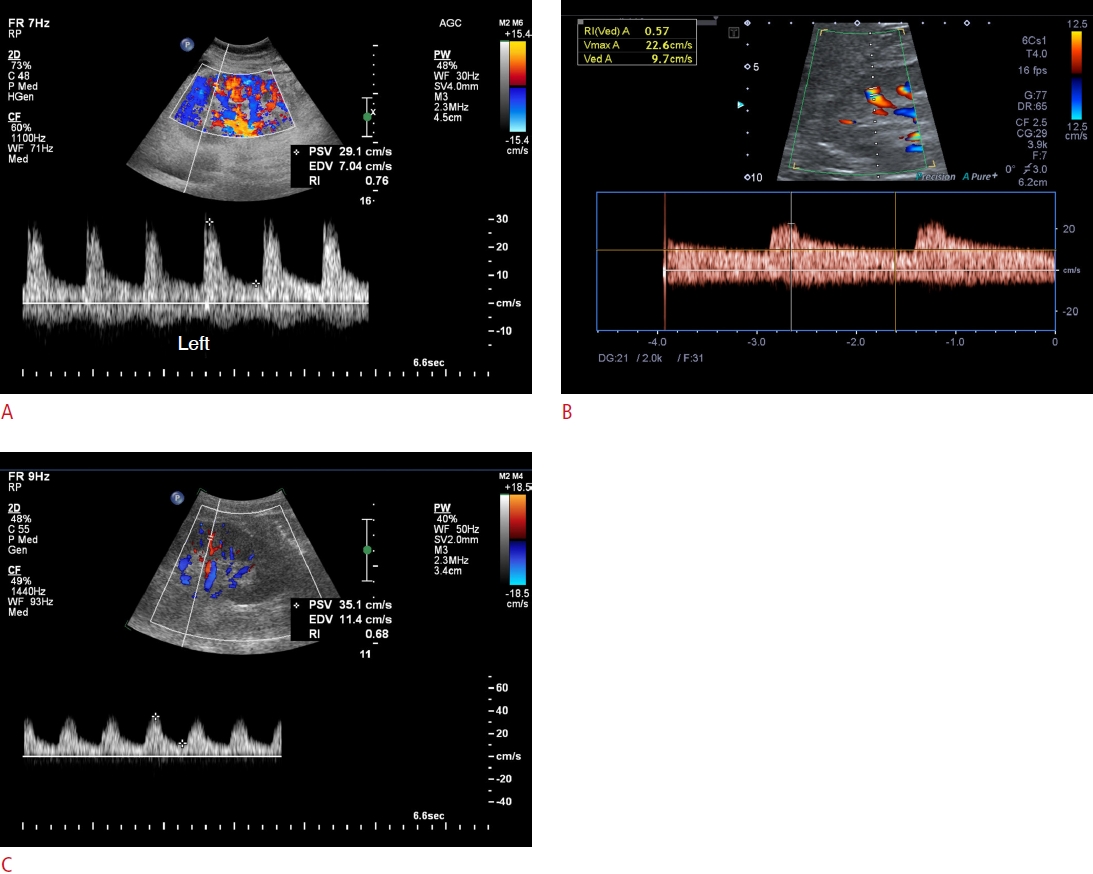 Fig. 2.ROC curve of the RI for diagnosing NDKD.The AUC is 0.759 (P=0.001) and the RI has a sensitivity of 57.1% (95% CI, 28.9% to 82.3%) and specificity of 81.0% (95% CI, 58.1% to 94.6%) for diagnosing NDKD, using a cutoff value of ≤0.69. ROC, receiver operating characteristic; RI, resistive index; NDKD, non-diabetic kidney disease; AUC, area under the ROC curve; CI, confidence interval.
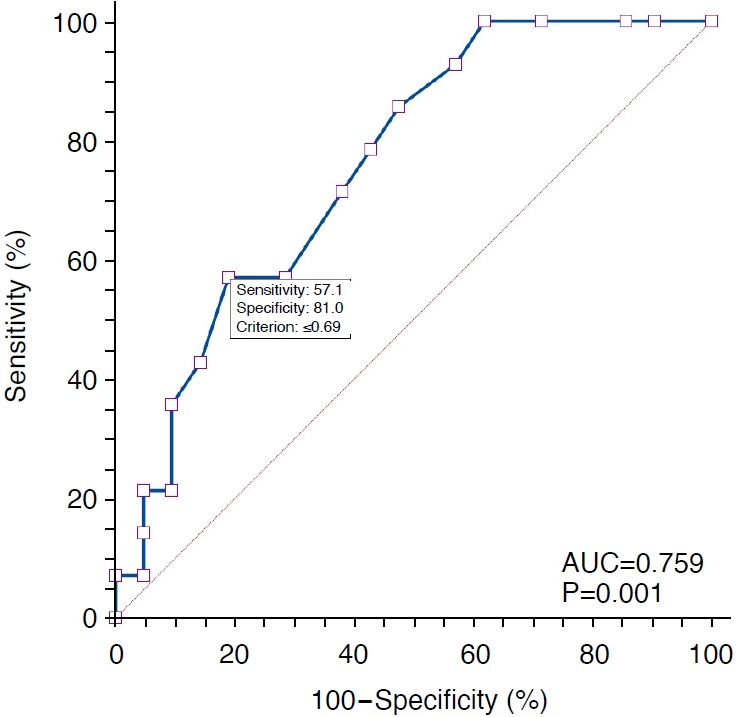 Fig. 3.Table 1.Histologic diagnoses in the NDKD group (n=30)
Table 2.Comparison of renal Doppler US findings between the DN and NDKD groups |



 Print
Print facebook
facebook twitter
twitter Linkedin
Linkedin google+
google+
 Download Citation
Download Citation PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI




