AbstractThe evolution of ultrasound (US) techniques has greatly improved the evaluation of many parameters in dialysis vascular access, which is typically achieved through an arteriovenous fistula (AVF) or graft (AVG). These techniques include grayscale B-mode, color Doppler, power Doppler, spectral Doppler, non-Doppler US flow imaging techniques, contrast-enhanced US, and elastography. In conjunction with a patient’s medical history and physical examination, US provides crucial information about the native vascular bed prior to the surgical creation of an arteriovenous anastomosis. It also tracks the maturation progress of the newly created AVF or AVG and aids in diagnosing potential complications of the vascular access. These complications include thrombosis, steal syndrome, aneurysms, pseudoaneurysms, hematomas, infection, ischemic neuropathy, exacerbation of preexisting congestive heart failure, and stenosis.
Globally, approximately 2 million patients have been diagnosed with end-stage renal disease (ESRD), with an annual reported incidence of nearly 100,000 cases [1]. Patients with ESRD require some form of renal replacement therapy, with hemodialysis typically being the preferred treatment. Hemodialysis is commonly performed by placing two large-bore needles into a vascular access point in the upper extremity. Simply put, blood is drawn into the dialysis machine through one needle access, cleaned, and then returned to the patient through the second needle access [2].
Long-term hemodialysis typically necessitates vascular access in the upper extremity, either through an arteriovenous fistula (AVF) or an arteriovenous graft (AVG). Central venous catheters can also provide vascular access for patients undergoing hemodialysis, but these devices are associated with a substantial risk of infection, which increases morbidity and mortality. Regardless of the type of vascular access used, it is necessary to conduct surveillance through physical examination and imaging to identify potential complications and access failure. Ultrasonography (US) is a valuable tool for preoperative evaluation of the arterial and venous vasculature in the upper extremity [1]. This manuscript discusses the importance of multiparametric US in evaluating AVF and AVG.
AVFs are the preferred method for hemodialysis vascular access, compared to AVGs and central venous catheters. This is due to the lower risk of thrombosis and infection associated with AVFs, as well as their longer patency duration [1]. An AVF is surgically created by connecting a native vein and artery using an end-to-end, end-to-side (vein-to-artery), or side-to-side anastomosis. This is typically done on the non-dominant wrist or upper arm, provided the vascular anatomy on that side is suitable (Fig. 1). However, AVFs have some disadvantages, including a longer time to maturation (4-6 weeks) compared to AVGs and a higher risk of maturation failure. The most common types of AVFs are radiocephalic and brachiocephalic fistulas. The brachial artery and basilic vein can also be used, but creating this type of AVF requires a second transposition procedure [1].
An AVG is a prosthetic conduit consisting of polytetrafluoroethylene or a bovine carotid artery graft, placed under the skin to establish a communication between an artery and a vein. AVGs require a shorter maturation period than AVFs (2-4 weeks) and can be utilized with smaller-caliber veins. This makes them a suitable choice for patients with limited venous access options. However, AVGs carry a higher risk of thrombosis and infectious complications compared to AVFs [1].
Central venous catheters serve as a temporary solution for vascular access. However, due to the high risk of infection associated with these catheters, it is advisable to replace them promptly with an upper-extremity AVG or AVF [3].
Prior to fistula creation, a proper assessment of the arterial and venous system should be conducted. This evaluation should include a physical examination and color Doppler US, as well as computed tomography, magnetic resonance imaging, or digital subtraction angiography, as clinically warranted. In combination with the patient’s history and physical exam, these examinations should allow clinicians to perform an adequate evaluation of the anatomical and functional features of the vasculature to determine which patients are suitable candidates for surgical AVF or AVG creation [1,4].
High-frequency linear transducers (>7.5 Hz) should be utilized for the US examination. The examination room should be warm, and a substantial amount of body-temperature gel should be applied to prevent vasospasm [1,5]. Initially, grayscale, color Doppler, and pulsed-wave Doppler imaging should be used to examine the arteries and veins of the non-dominant arm. If no suitable vessels for fistula formation are found, the dominant arm should then be examined [6]. The arterial examination should include the subclavian, axillary, brachial, radial, and ulnar arteries. Each artery should be evaluated for its patency, course, and any presence of obstructive atherosclerotic disease. The depth of the artery from the skin should also be measured (Fig. 2). The internal diameter of the artery intended for fistula creation should be at least 2 mm. Functional characteristics of the arteries should be assessed using spectral Doppler imaging, including the evaluation of blood flow and the capacity of the vessel to dilate. Blood flow can be determined by calculating the vessel’s diameter and the mean peak systolic velocity (PSV) (cm/s) in the longitudinal plane. Previous studies have shown that a radial artery flow volume greater than 50 mL/min is associated with successful radiocephalic AVF formation. Conversely, a preoperative radial artery flow volume of less than 20 mL/min is linked to an increased risk of AVF failure within the first 8 months. The capacity of arteries to dilate can be estimated using the reactive hyperemia test [1,5].
Preoperative venous mapping encompasses the examination of both superficial and deep venous systems, extending from the wrist to the visible central veins. It is crucial to assess all veins for patency through compression, as they will be noncompressible if they are partially or fully thrombosed. The vein lumen is scrutinized for any echogenic material representing thrombus or stenoses. The thickness of the wall and the diameter of the vessel are also evaluated. For AVF creation, it is recommended that the venous diameter be at least 2.5 mm with a tourniquet and 2 mm without one. The course of the veins is mapped, and ideally, a vein used for outflow should have a reasonably linear path. Another important parameter to examine is the depth of the vein from the skin, which should be less than 5 to 6 mm for a length of 10 cm or more to ensure successful needle cannulation [1,5]. The visualization of central veins using US is limited. Therefore, the proximal segments of the subclavian vein, innominate vein, and superior vena cava are best assessed with digital subtraction venography. This technique employs either iodine-based contrast or carbon dioxide as a contrast agent, depending on the clinical situation [1,5].
Ideally, US examinations of AVFs and AVGs should be conducted more than 24 hours after the last dialysis session [7]. In these US examinations, the blood flow volume is calculated within the graft for patients with AVGs and within the brachial artery for patients with native AVFs, regardless of the subtype [7]. For a precise blood flow calculation, three consecutive measurements are recommended, except in patients with arrhythmia, where five measurements are beneficial to account for potential variability in cardiac output [8]. Most published studies suggest that an extracorporeal circuit/blood flow greater than 500 mL/min, coupled with a diameter larger than 4 mm in the venous outflow tract, represent unremarkable findings in patients with AVFs [9].
During US examinations, it is important to note any collateral vasculature near the access site. This can increase the risk of recirculation phenomena (Fig. 3) as well as extraluminal pathologies such as hematoma and subcutaneous edema [10]. Finally, to achieve successful cannulation, the tips of the two large-gauge needles must be placed at a specific distance apart to prevent recirculation phenomena. Consequently, a straight venous segment of at least 4 cm in the cannulation zone is necessary for appropriate two-needle access [7].
Although AVFs are the preferred method for dialysis access, they are unsuitable for proper use in approximately 20% to 60% of cases due to inadequate maturation (Fig. 4) [11,12]. Maturation is the process through which a fistula develops the necessary characteristics for successful two-needle cannulation and subsequent high-quality hemodialysis. The "rule of 6’s" (Table 1) is a commonly used guideline for this process: a mature fistula should have a diameter of at least 6 mm and clear margins during tourniquet placement within 6 weeks of surgical creation. Additionally, the access vessel depth should be less than 6 mm, and blood flow should exceed 600 mL/min (Fig. 5) [13]. Recent studies have also associated maturation with suitable hemodynamics, characterized by brisk forward flow and the absence of large venous collateral branches that could divert flow from the upper extremity vascular access [14]. In this context, vascular remodeling refers to the gradual increase in diameter and blood flow, which in turn increases the capacity of the accessed vein and venous outflow system [15]. However, one potential limitation of US maturation assessment is the lack of a clearly defined optimal location for blood flow measurements [16].
Clinical maturation refers to the capacity of a fistula to be used for hemodialysis with two large-gauge needles for at least 75% of a patient’s dialysis sessions over a 2-week period [17]. Previous studies have demonstrated that clinical maturation can be accurately predicted by palpation of a thrill in the cannulation zone during physical examination in 72% to 80% of patients [18]. However, physical examination is less effective in predicting clinical maturation in patients with thicker subcutaneous fat and in those with AVFs exhibiting delayed maturation [19]. These patients may benefit from serial US examinations to precisely monitor the maturation process.
Most AVF maturation failures can be attributed to underlying venous outflow stenosis. This condition gradually reduces blood flow in the outflow vein, which in turn alters the AVF pressure, leading to a failure to mature and eventually resulting in complete occlusive thrombosis of the upper-extremity access [20]. In a single-institution retrospective review, duplex US was found to offer better sensitivity than physical examination in identifying immature AVFs, thereby highlighting the potential need for reintervention [21]. In a separate study, the assessment of fistula maturation using Doppler US was found to correlate well with clinically significant stenosis observed on invasive fistulograms, which were used as a reference standard [22]. This study also found that most de novo stenoses in the maturation process appeared immediately after the arterialization of the superficial veins. These stenoses were primarily due to intimal hyperplasia, and Doppler US was effective in identifying them [23]. Previous studies have recommended conducting Doppler US examinations at 1, 2, and 6 weeks after surgical creation of the AVF/AVG, particularly if problems are detected during physical examinations at outpatient visits [22,23].
The necessity of maintaining functioning vascular access and managing any related complications are the primary sources of morbidity in patients with ESRD [24]. AVFs are associated with lower complication rates than AVGs and central venous dialysis catheters. The most common complications of AVFs include thrombosis, steal syndrome, aneurysm/pseudoaneurysm/hematoma formation, infection, ischemic neuropathy, exacerbation of congestive heart failure, stenosis, and lymphedema [25]. Early diagnosis and proper treatment of these conditions may improve outcomes among patients with ESRD [26].
Thrombosis is a common late fistula complication that must be treated endovascularly to prevent vascular access abandonment. AVFs are associated with lower rates of thrombotic events than AVGs and central venous catheters [27]. The most common pathophysiological cause of fistula thrombosis is venous neointimal proliferation [28].
Patients with recurrent thrombotic episodes may need to be evaluated for an underlying hypercoagulable state. This could potentially be caused by homozygosity or heterozygosity for factor V Leiden, ATIII levels that are less than 60% of normal values, or a deficiency in proteins C and S. Chronic kidney disease can also lead to coagulation abnormalities, such as elevated levels of factor VIII, fibrinogen, D-dimer, and homocysteine. It may also cause a reduction in thrombin clotting time and/or the presence of antiphospholipid antibodies. Venous outflow stenosis, which can lead to stasis in the access, can also predispose patients to thrombosis in upper extremity vascular access [29,30].
Color Doppler US is the preferred noninvasive method for diagnosing thrombosis. Typically, a clotted vein appears filled with a solid material of mixed echogenicity, is noncompressible, and does not react to dynamic tests such as the Valsalva maneuver. In the early stages, the thrombus echogenicity is low, but it increases over time as the thrombus becomes more chronic. Color Doppler US often reveals a lack of flow signals, and a persistent high-resistance triphasic trace is frequently observed within the artery that supplies the fistula (Figs. 6, 7) [29]. The thrombus may be either completely or partially occlusive (Fig. 8). Patients with acute thrombosis in AVFs usually experience sudden pain in the affected upper extremity [25]. Acute thrombosis requires immediate pharmacomechanical thrombectomy to prevent its progression into chronic thrombotic disease, which could result in permanent loss of vascular access in the upper extremity (Fig. 9). On color Doppler US, a chronic thrombus appears hyperechoic and calcified. Chronic thrombosis narrows the vessel diameter and results in the loss of a spectral curve. In cases of acute or subacute thrombosis, subcutaneous edema may also be detected (Figs. 10, 11) [29].
Dialysis access-related steal syndrome (DASS) is an uncommon but recognized complication that most commonly affects smokers, patients with diabetes, and elderly individuals (aged 80 years and older). DASS more frequently occurs in patients with AVGs than in those with AVFs [31]. Among patients with AVFs, steal syndrome is more common in those whose access is supplied by the brachial artery [32]. Symptomatic ischemia in the distal upper extremity is seen in 10% to 25% of patients with brachiocephalic and brachiobasilic vascular access, while only 1% to 2% of patients with radiocephalic AVFs experience symptomatic ischemia [33]. Typically, patients who experience this complication begin showing symptoms during their dialysis session.
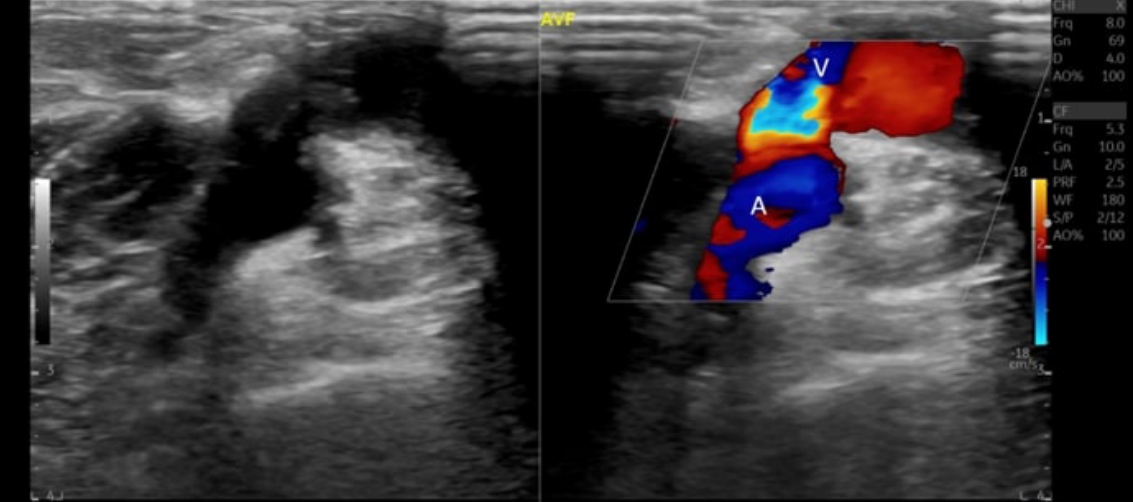
DASS is a result of arterial insufficiency that occurs distal to the AVF. This condition implies that the bulk of the arterial blood supply is directed into the anastomosis instead of the distal artery. This diversion can lead to hand ischemia, hypoxia, and necrosis [1]. In parallel, an increase in flow occurs within the fistula along with bidirectional flow in the distal artery. These phenomena are clearly depicted on US images [27,34,35].
Treatment of this condition presents challenges, including a high risk of finger and forearm amputation. The preferred method for managing hand ischemia while preserving the AVF is the distal revascularization-interval ligation procedure [31]. However, in severe instances, it may be necessary to completely abandon the access through surgical ligation.
An important category of complications related to upper extremity vascular access involves vein wall injuries that result in aneurysms, pseudoaneurysms, and hematomas [29]. An aneurysm is a condition where a portion of the artery wall weakens, affecting all three layers of the vessel wall and causing abnormal dilation. A pseudoaneurysm is essentially a contained vessel leak that occurs due to iatrogenic trauma in situations involving repetitive access punctures [36]. Unlike hematomas, pseudoaneurysms maintain a connection with the vessel lumen [29].
Color Doppler US is essential for the evaluation of a focal mass at the upper extremity vascular access site. This modality enables the differentiation of aneurysms, pseudoaneurysms, and hematomas from an acutely thrombosed bulky vascular access, also known as a pseudomass. Pseudoaneurysms often manifest as a pulsatile mass. When examined with color Doppler US, they exhibit the distinctive "yin-yang sign" or swirling flow pattern, as well as bidirectional flow that has been characterized as displaying a "to-and-fro pattern" at the neck of the pseudoaneurysm [1,29]. Hematomas present as a heterogeneous fluid collection, primarily extending into the adjacent musculature and subcutaneous tissues (Fig. 12) [29]. In contrast, aneurysms appear as a saccular dilation of all layers of the affected vasculature. The complexity of endovascular and surgical treatment options is beyond the scope of this article [29,37].
Although it has been reported that the infection rate of AVFs is one-tenth the infection rate of AVGs [38], infections still represent up to 20% of all complications associated with AVFs [25,38]. These infections can vary in severity, from perivascular cellulitis and edema to more serious conditions such as abscesses that necessitate percutaneous drainage (Fig. 13) [38]. Symptoms may include redness or pain at the site of vascular access, along with systemic signs such as fever and chills [29]. Infection diagnosis is typically clinical, and US plays a limited role. It is primarily used to verify graft patency and functionality, as well as to identify any drainable abscesses [29].
Ischemic polyneuropathy is a rare complication of AVF that typically develops rapidly within the first few hours following AVF creation [29]. This condition predominantly affects female and diabetic patients with preexisting neuropathy [29]. The clinical symptoms include weakness in the distal muscle groups, intense pain, and paresthesia. The affected hand is usually warm, and a palpable radial pulse or a detectable Doppler US signal is often present [39]. The etiology of this complication is believed to be acute limb ischemia, which occurs when arterial blood is diverted away from the distal extremity after the creation of an AVF, leading to multiple axonal loss mononeuropathies in these patients [39]. US does not play a significant role in diagnosing ischemic neuropathy, which is primarily determined based on clinical parameters.
AVFs increase cardiac output, potentially leading to detrimental effects on cardiac function, especially in the context of preexisting heart disease [29]. Research has shown that the creation of AVFs in patients with ESRD can lead to increased instances of left ventricular hypertrophy, pulmonary hypertension, right ventricular dysfunction, coronary artery disease, and valvular heart disease [40,41].
Stenotic disease in AVFs and AVGs is characterized by the narrowing of the vessel lumen due to neointimal hyperplasia or a partially obstructive thrombus [29]. This narrowing results in elevated venous pressures and poor flow rates during dialysis (Fig. 14) [29]. The risk of thrombosis generally increases with the degree of stenosis. In AVFs, stenosis most frequently occurs in the juxta-anastomotic segment (80%). This type of stenosis impacts the initial 2 cm of the draining vein near the arteriovenous anastomosis, which can ultimately lead to early fistula failure (Figs. 15, 16) [29]. In the case of AVGs, stenoses predominantly occur at the venous anastomoses and occasionally at the draining vein, central vein, feeding artery, or within the graft lumen [1]. Signs of an underlying stenosis may include AVF/AVG dysfunction, difficulties with cannulation zone puncture, pain at the fistula site, and elevated venous pressures.
Color and spectral Doppler techniques are the most accurate noninvasive methods for diagnosing stenoses, as they can identify lumen narrowing and facilitate measurements of PSV. US criteria for stenosis encompass morphological and hemodynamic factors, as well as changes in velocity. Morphologically, arterial stenosis is deemed critical based on a 60% to 75% vessel narrowing, while venous stenosis is considered critical with a 50% reduction in vessel diameter [29]. Hemodynamically, aliasing and color turbulent (variant) flow on color Doppler US suggest underlying stenotic disease. On spectral Doppler US, PSV values exceeding 200 to 250 cm/s at the tributary artery or over 300 to 400 cm/s in the outflow vein are direct indicators of stenosis [29]. Stenosis over 50% is characterized by a 3:1 PSV ratio measured at the stenosis and 2 cm cephalad, as well as a doubling of PSV in the draining vein (Figs. 17, 18) [1,29]. Published guidelines (Table 2) have established basic criteria for the presence of significant stenosis in upper extremity vascular access, which include a narrowing of the vascular lumen over 50% and a PSV ratio (the ratio between PSV in the stenotic and pre-stenotic area) over 2. Additionally, at least one of the following criteria must be present: blood flow less than 500 mL/min in AVFs or less than 600 mL/min in AVGs, a blood flow reduction of more than 25% in cases of blood flow less than 1,000 mL/min, and/or a residual patent diameter of the examined vessel less than 2 mm [42]. For AVGs, when no additional criteria are present, the detected stenosis is classified as borderline. In these instances, a repeat scan may be considered after 6 to 8 weeks [43]. A narrow graft diameter toward the arterial anastomosis may be associated with expected and unremarkable postoperative changes, and this may have the benefit of reducing the risk of steal phenomena leading to hand ischemia [7]. The treatment of stenotic disease is beyond the scope of this article and may involve angioplasty, including the use of drug-coated balloons, stent-graft placement, or surgical revision [29,36].
The term "multiparametric US" was introduced to describe the diverse aspects and complementary tools of US [44]. This umbrella term includes conventional anatomic techniques such as grayscale B-mode; the spectrum of Doppler techniques including color, power, and spectral Doppler; and recent advances such as non-Doppler flow visualization techniques, contrast-enhanced US, and elastography. The application of these various techniques has been demonstrated beneficial across a range of organs and clinical situations, including carotid atherosclerotic disease [45-47]. Table 3 outlines the future potential of multiparametric US in the context of vascular access assessment. Advanced techniques may help overcome obstacles and serve as a problem-solving tool in complex cases. Fig. 19 provides a representative example of the use of elastography in a case of AVF thrombosis.
A functional venous access, in the form of an AVF or AVG, enhances the quality of life among patients with ESRD by facilitating safe and effective hemodialysis. However, upper extremity vascular accesses require endovascular maintenance due to the risk of failure and potential complications. US plays a pivotal role not only in preoperative planning and mapping of the upper extremity vasculature, but also in monitoring the function and patency of AVFs and AVGs. This allows clinicians to detect complications and impending failure early. These US findings substantially contribute to the management of the ESRD patient population. Utilizing various blood-flow visualization techniques, US is the noninvasive imaging modality of choice for evaluating upper extremity vascular accesses.
NotesAuthor Contributions Conceptualization: Michas V, Taghipour M, Papachristodoulou A, Rafailidis V, Michell H, Prassopoulos P. Data acquisition: Michas V, Taghipour M, Sidiropoulou M, Partovi S, Cokkinos D, Rafailidis V, Gadani S, Prassopoulos P. Data analysis or interpretation: Michas V, Taghipour M, Partovi S, Rafailidis V, Gill A, Michell H, Prassopoulos P. Drafting of the manuscript: Michas V, Taghipour M, Sidiropoulou M, Rafailidis V, Gadani S, Gill A, Prassopoulos P. Critical revision of the manuscript: Michas V, Taghipour M, Papachristodoulou A, Partovi S, Cokkinos D, Rafailidis V, Michell H, Prassopoulos P. Approval of the final version of the manuscript: all authors. References1. Athanasiou S, Cokkinos DD, Antypa EG, Kalogeropoulos I. Arteriovenous fistulas and grafts for haemodialysis: what are they, how to examine them with ultrasound for preoperative mapping, monitoring and management of complications. Hellenic J Radiol 2020;5:36–47.
3. Quencer KB, Arici M. Arteriovenous fistulas and their characteristic sites of stenosis. AJR Am J Roentgenol 2015;205:726–734.
4. Vallespin J, Meola M, Ibeas J. Upper limb anatomy and preoperative mapping. J Vasc Access 2021;22:9–17.
5. Zamboli P, Fiorini F, D'Amelio A, Fatuzzo P, Granata A. Color Doppler ultrasound and arteriovenous fistulas for hemodialysis. J Ultrasound 2014;17:253–263.
6. Stegmayr B, Willems C, Groth T, Martins A, Neves NM, Mottaghy K, et al. Arteriovenous access in hemodialysis: a multidisciplinary perspective for future solutions. Int J Artif Organs 2021;44:3–16.
7. Chytilova E, Jemcov T, Malik J, Pajek J, Fila B, Kavan J. Role of Doppler ultrasonography in the evaluation of hemodialysis arteriovenous access maturation and influencing factors. J Vasc Access 2021;22:42–55.
8. Grosser S, Kreymann G, Kuhns A, Greten H. Color-coded duplex sonography in primary diagnosis, monitoring and prognostic evaluation of fibrinolytic and conservative therapy of thrombophlebitis. Vasa Suppl 1991;32:187–190.
9. Kotze CW, Ahmed IG. Etiology and pathogenisis of aortic aneurysm. In: Grundmann R, ed. Etiology, pathogenesis and pathophysiology of aortic aneurysms and aneurysm rupture London: InTech, 2011.
10. Beathard GA, Arnold P, Jackson J, Litchfield T; Physician Operators Forum of RMS Lifeline. Aggressive treatment of early fistula failure. Kidney Int 2003;64:1487–1494.
11. Dember LM, Imrey PB, Beck GJ, Cheung AK, Himmelfarb J, Huber TS, et al. Objectives and design of the hemodialysis fistula maturation study. Am J Kidney Dis 2014;63:104–112.
12. Dember LM, Beck GJ, Allon M, Delmez JA, Dixon BS, Greenberg A, et al. Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: a randomized controlled trial. JAMA 2008;299:2164–2171.
13. Vascular Access Work Group. Clinical practice guidelines for vascular access. Am J Kidney Dis 2006;48 Suppl 1:S176–S247.
14. Etkin Y, Talathi S, Rao A, Akerman M, Lesser M, Mussa FF, et al. The role of duplex ultrasound in assessing AVF maturation. Ann Vasc Surg 2021;72:315–320.
16. Robbin ML, Greene T, Allon M, Dember LM, Imrey PB, Cheung AK, et al. Prediction of arteriovenous fistula clinical maturation from postoperative ultrasound measurements: findings from the hemodialysis fistula maturation study. J Am Soc Nephrol 2018;29:2735–2744.
17. Singh M, Mahapatra HS, Pursnani L, Muthukumar B, Neeraj Anant I, Kumar A, et al. Study on prediction of arterio-venous fistula maturation by flow mediated dilatation and AVF blood flow. J Vasc Access 2023;24:443–451.
18. Ferring M, Henderson J, Wilmink T. Accuracy of early postoperative clinical and ultrasound examination of arteriovenous fistulae to predict dialysis use. J Vasc Access 2014;15:291–297.
19. Davidson I, Chan D, Dolmatch B, Hasan M, Nichols D, Saxena R, et al. Duplex ultrasound evaluation for dialysis access selection and maintenance: a practical guide. J Vasc Access 2008;9:1–9.
20. Rajabi-Jaghargh E, Banerjee RK. Combined functional and anatomical diagnostic endpoints for assessing arteriovenous fistula dysfunction. World J Nephrol 2015;4:6–18.
21. Caputo BC, Leong B, Sibona A, Jhajj S, Kohne C, Gabel J, et al. Arteriovenous fistula maturation: physical exam versus flow study. Ann Vasc Surg 2021;77:16–24.
22. Grogan J, Castilla M, Lozanski L, Griffin A, Loth F, Bassiouny H. Frequency of critical stenosis in primary arteriovenous fistulae before hemodialysis access: should duplex ultrasound surveillance be the standard of care? J Vasc Surg 2005;41:1000–1006.
23. Chandra AP, Dimascio D, Gruenewald S, Nankivell B, Allen RD, Swinnen J. Colour duplex ultrasound accurately identifies focal stenoses in dysfunctional autogenous arteriovenous fistulae. Nephrology (Carlton) 2010;15:300–306.
24. Manns B, Tonelli M, Yilmaz S, Lee H, Laupland K, Klarenbach S, et al. Establishment and maintenance of vascular access in incident hemodialysis patients: a prospective cost analysis. J Am Soc Nephrol 2005;16:201–209.
25. Stolic R. Most important chronic complications of arteriovenous fistulas for hemodialysis. Med Princ Pract 2013;22:220–228.
26. Rosas SE, Feldman HI. Synthetic vascular hemodialysis access versus native arteriovenous fistula: a cost-utility analysis. Ann Surg 2012;255:181–186.
27. Al-Jaishi AA, Liu AR, Lok CE, Zhang JC, Moist LM. Complications of the arteriovenous fistula: a systematic review. J Am Soc Nephrol 2017;28:1839–1850.
28. Bonatti J, Oberhuber A, Schachner T, Zou Y, Hammerer-Lercher A, Mittermair R, et al. Neointimal hyperplasia in coronary vein grafts: pathophysiology and prevention of a significant clinical problem. Heart Surg Forum 2004;7:72–87.
29. Meola M, Marciello A, Di Salle G, Petrucci I. Ultrasound evaluation of access complications: thrombosis, aneurysms, pseudoaneurysms and infections. J Vasc Access 2021;22:71–83.
30. Salmela B, Hartman J, Peltonen S, Alback A, Lassila R. Thrombophilia and arteriovenous fistula survival in ESRD. Clin J Am Soc Nephrol 2013;8:962–968.
31. Mwipatayi BP, Bowles T, Balakrishnan S, Callaghan J, Haluszkiewicz E, Sieunarine K. Ischemic steal syndrome: a case series and review of current management. Curr Surg 2006;63:130–135.
32. Lazarides MK, Staramos DN, Kopadis G, Maltezos C, Tzilalis VD, Georgiadis GS. Onset of arterial 'steal' following proximal angioaccess: immediate and delayed types. Nephrol Dial Transplant 2003;18:2387–2390.
33. Tordoir JH, Dammers R, van der Sande FM. Upper extremity ischemia and hemodialysis vascular access. Eur J Vasc Endovasc Surg 2004;27:1–5.
34. Yilmaz C, Ozcan K, Erkan N. Dialysis access-associated steal syndrome presenting as bidirectional flow at duplex Doppler ultrasound. AJR Am J Roentgenol 2009;193:W568.
35. Malik J, Slavikova M, Maskova J. Dialysis access-associated steal syndrome: the role of ultrasonography. J Nephrol 2003;16:903–907.
36. Saeed F, Kousar N, Sinnakirouchenan R, Ramalingam VS, Johnson PB, Holley JL. Blood loss through AV fistula: a case report and literature review. Int J Nephrol 2011;2011:350870.
37. Mancini A, Castriotta G, Angelini P, Bozzi M, Giancaspro V, La Raia E, et al. Brachial artery pseudoaneurysm: a rare but serious complication in hemodialysis patients with arteriovenous fistula. G Ital Nefrol 2017;34:44–53.
38. Saxena AK, Panhotra BR, Al-Mulhim AS. Vascular access related infections in hemodialysis patients. Saudi J Kidney Dis Transpl 2005;16:46–71.
40. Alkhouli M, Sandhu P, Boobes K, Hatahet K, Raza F, Boobes Y. Cardiac complications of arteriovenous fistulas in patients with end-stage renal disease. Nefrologia 2015;35:234–245.
41. Basile C, Lomonte C, Vernaglione L, Casucci F, Antonelli M, Losurdo N. The relationship between the flow of arteriovenous fistula and cardiac output in haemodialysis patients. Nephrol Dial Transplant 2008;23:282–287.
42. Ibeas J, Roca-Tey R, Vallespin J, Moreno T, Monux G, Marti-Monros A, et al. Spanish clinical guidelines on vascular access for haemodialysis. Nefrologia 2017;37 Suppl 1:1–191.
43. Tuka V, Slavikova M, Krupickova Z, Mokrejsova M, Chytilova E, Malik J. Short-term outcomes of borderline stenoses in vascular accesses with PTFE grafts. Nephrol Dial Transplant 2009;24:3193–3197.
44. Sidhu PS. Multiparametric ultrasound (MPUS) imaging: terminology describing the many aspects of ultrasonography. Ultraschall Med 2015;36:315–317.
45. Alexandratou M, Papachristodoulou A, Li X, Partovi S, Davidhi A, Rafailidis V, et al. Advances in noninvasive carotid wall imaging with ultrasound: a narrative review. J Clin Med 2022;11:6196.
Prior to surgical fistula creation, ultrasound mapping is used to estimate the diameter and depth of the vessels.In each instance, the measurements (shown in red boxes) denote the diameter (1) and the depth from the skin (2). In this case, the radial artery (arrow) (A), brachial artery (arrow) (B), and cephalic vein (arrow) (C) are all suitable for arteriovenous fistula (AVF) formation. However, the diameter of the cephalic vein (arrow) (D) is insufficient for AVF creation.
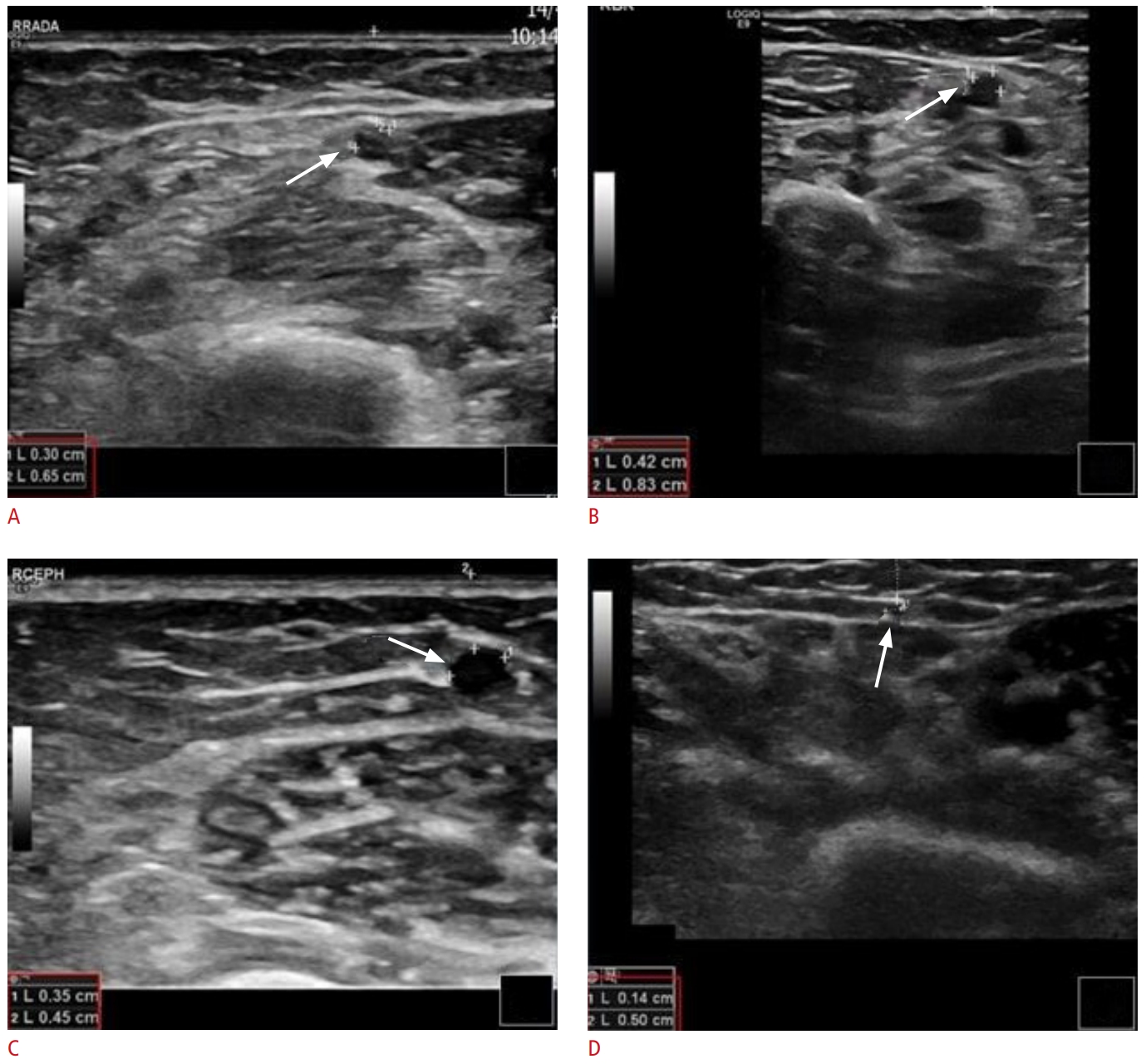 Fig. 2.Malfunctioning of arteriovenous fistula with recirculation phenomenon.A 79-year-old man with end-stage renal disease undergogind hemodialysis via a right radio-antecubital vein (an atypical Brescia-Cimino fistula). Grayscale, color, and spectral Doppler ultrasound (US) images reveal turbulent blood flow with significantly increased focal peak systolic velocity (A), suggestive of 50% to 99% stenosis of the venous outflow system. This is confirmed on a fistulogram (B, blue arrow) and subsequently treated with angioplasty (C, blue arrow). There is also evidence suggesting a prominent communicating peripheral vein (D-F, yellow arrows) diverting blood flow and thereby decreasing the flow rate of the vascular access. Corresponding to the US image, fistulograms show venous side branches originating from the cephalic vein and diverting flow (G-J, red arrows).
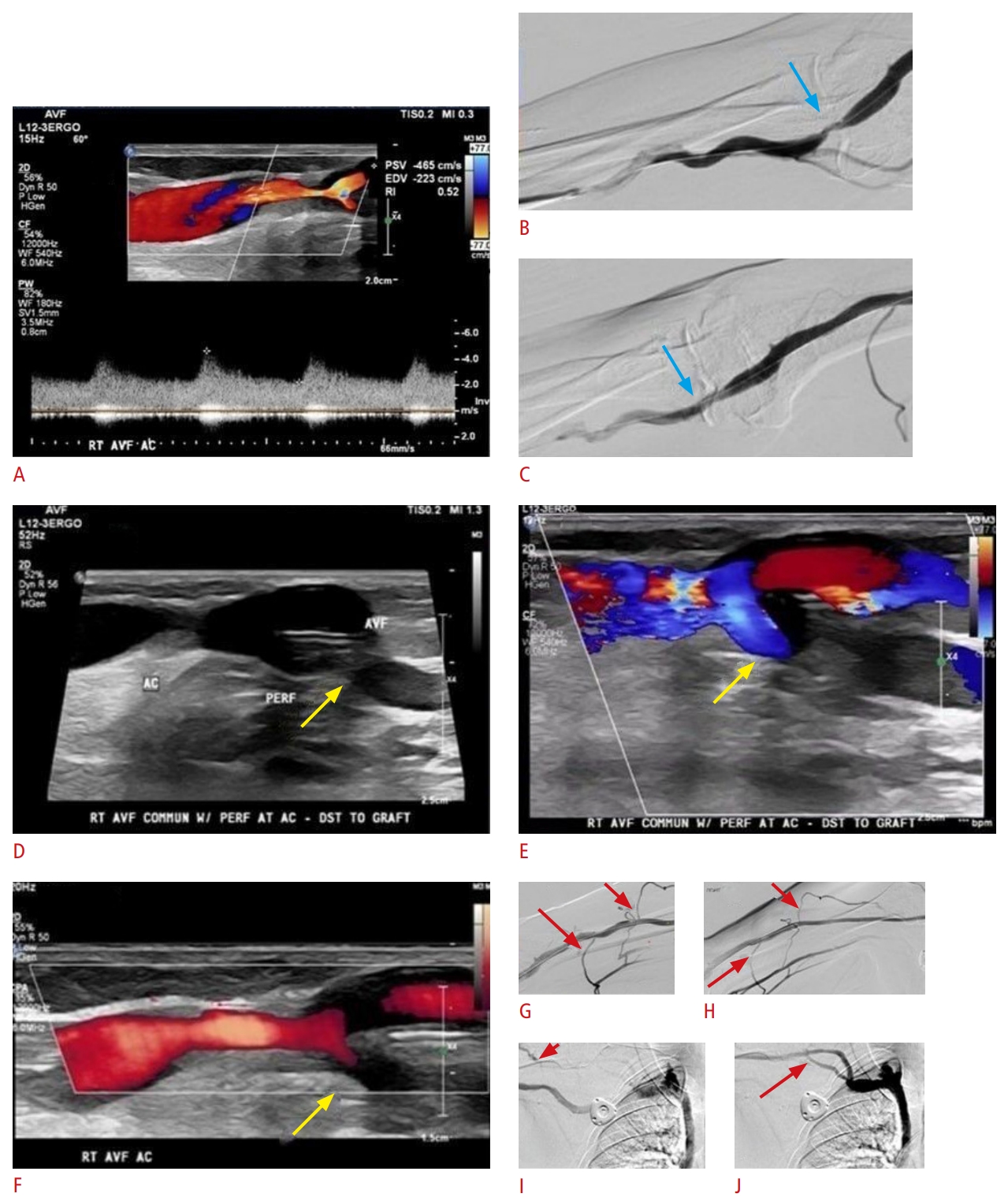 Fig. 3.Ultrasound findings in a fistula with inadequate maturation period.A recently surgically created arteriovenous fistula (AVF) (arrow) is shown. (A). Although the AVF appears patent (B), the blood flow remains low (flow volume, 25.9 mL/min; indicated by the red box) due to an inadequate maturation period (C) secondary to an inadequate maturation period (C). Within 6 weeks of surgical creation, a mature fistula should have a flow volume >600 mL/min.
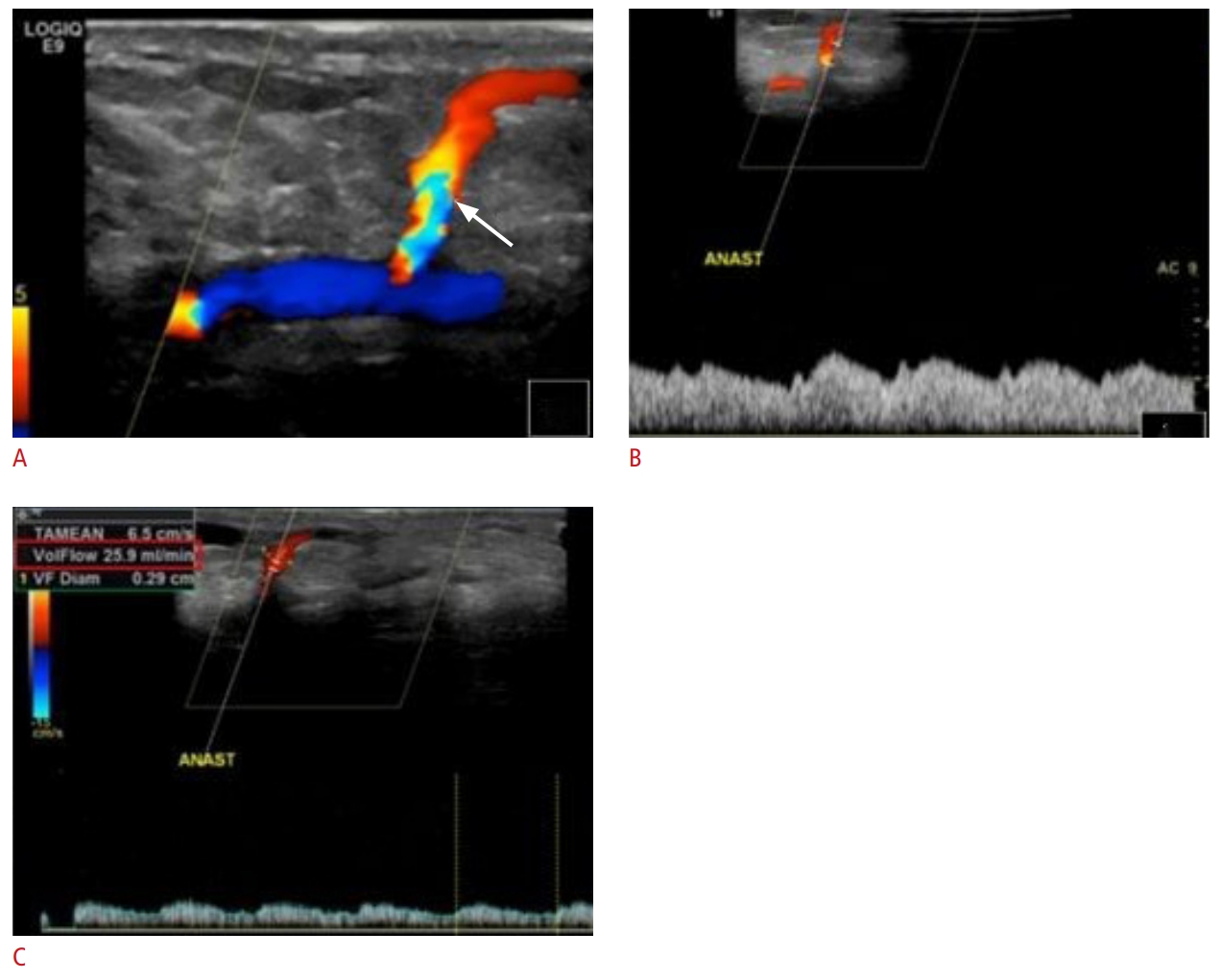 Fig. 4.Successful maturation of arteriovenous fistula.The blood flow volume of arteriovenous fistula is measured as 1,467 mL/min (indicated by the red box). A blood flow >600 mL/min is considered satisfactory.
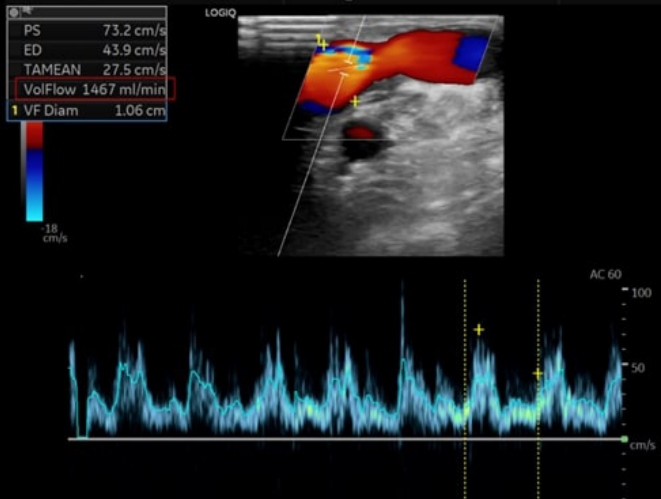 Fig. 5.Thrombosis of fistulas.A. Two separate fistulas (blue and red markings) were created at different time periods. B-mode ultrasound (US) demonstrates thrombosis (arrows) extending almost through the entire length of both fistulas (B-mode US with LOGIQ View scanning). B. The fistula, with only a small portion being patent (arrow), is unsuitable for hemodialysis. DIST, distally; PAT, patent; THR, thrombosed.
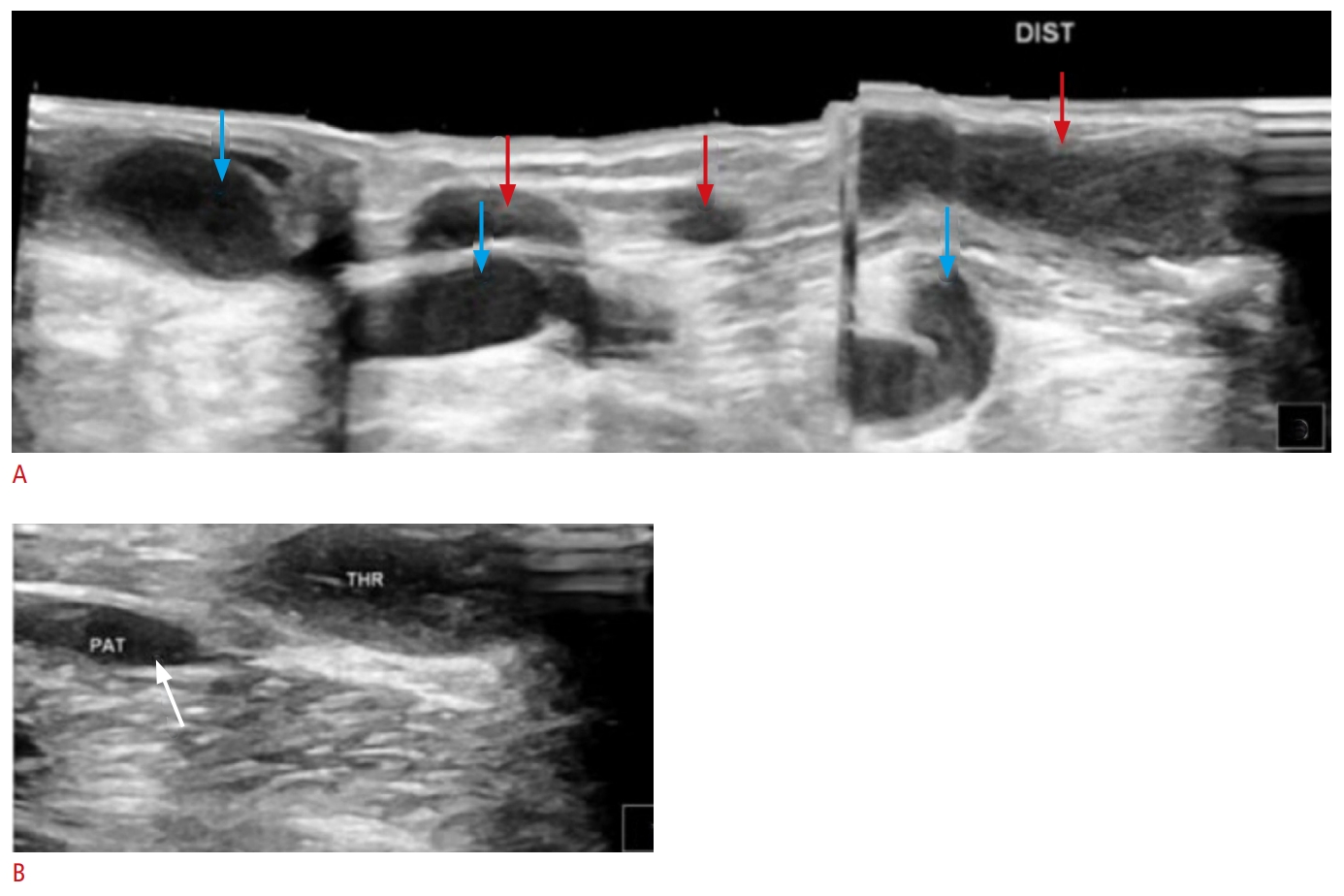 Fig. 6.Echogenic thrombotic material (noted by arrows) within an arteriovenous graft on B-mode ultrasound (US) (A, B) and color Doppler US (C).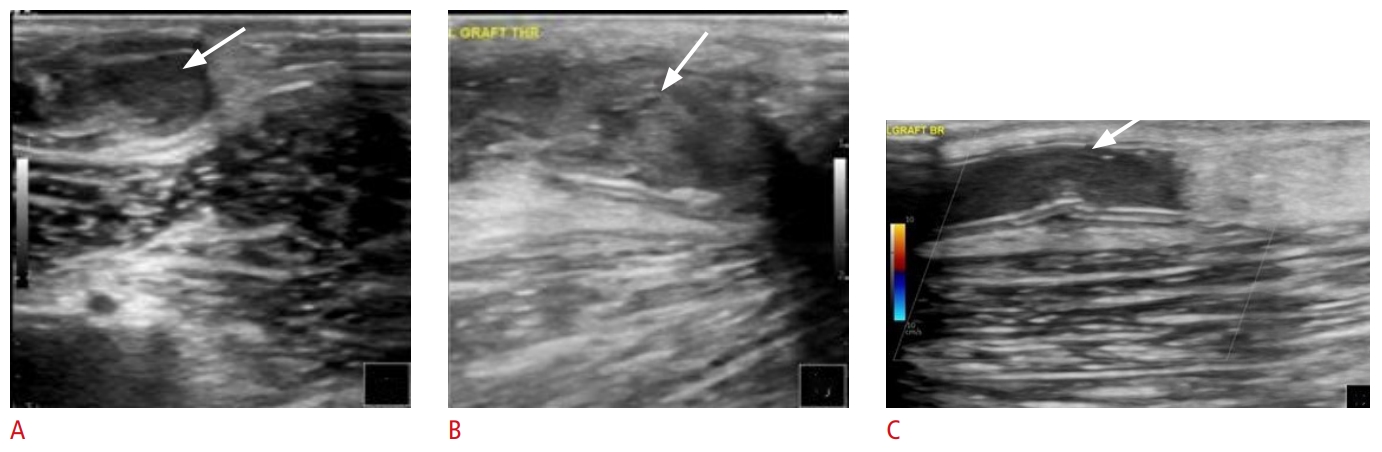 Fig. 7.Partial thrombosis of arteriovenous graft.A. Partially thrombosed arteriovenous graft is shown (arrow). B. However, the majority of the graft is patent, and blood flow is adequate for hemodialysis (flow volume, 2,993 mL/min; indicated by the red box).
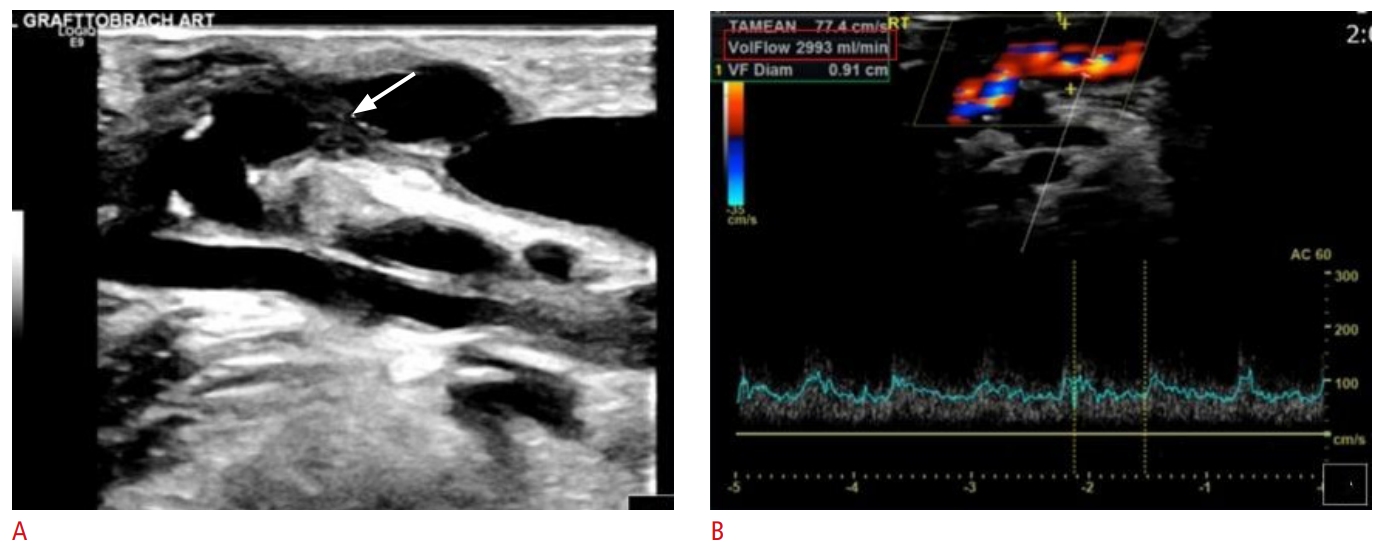 Fig. 8.Thrombosis of arteriovenous graft.A thrombosed arteriovenous graft (AVG) (A, arrow) is depicted undergoing pharmacomechanical thrombectomy. One week later, grayscale, color and Doppler US images reveal the AVG to be patent (B,C, arrow) with adeuquate blood flow (D: flow volume, 896.5 mL/min; indicated by the red box). Two prior AVGs (E, 1 and 2) had been chronically thrombosed, whereas the current AVG is patent (E, 3) and ready to be used for dialysis.
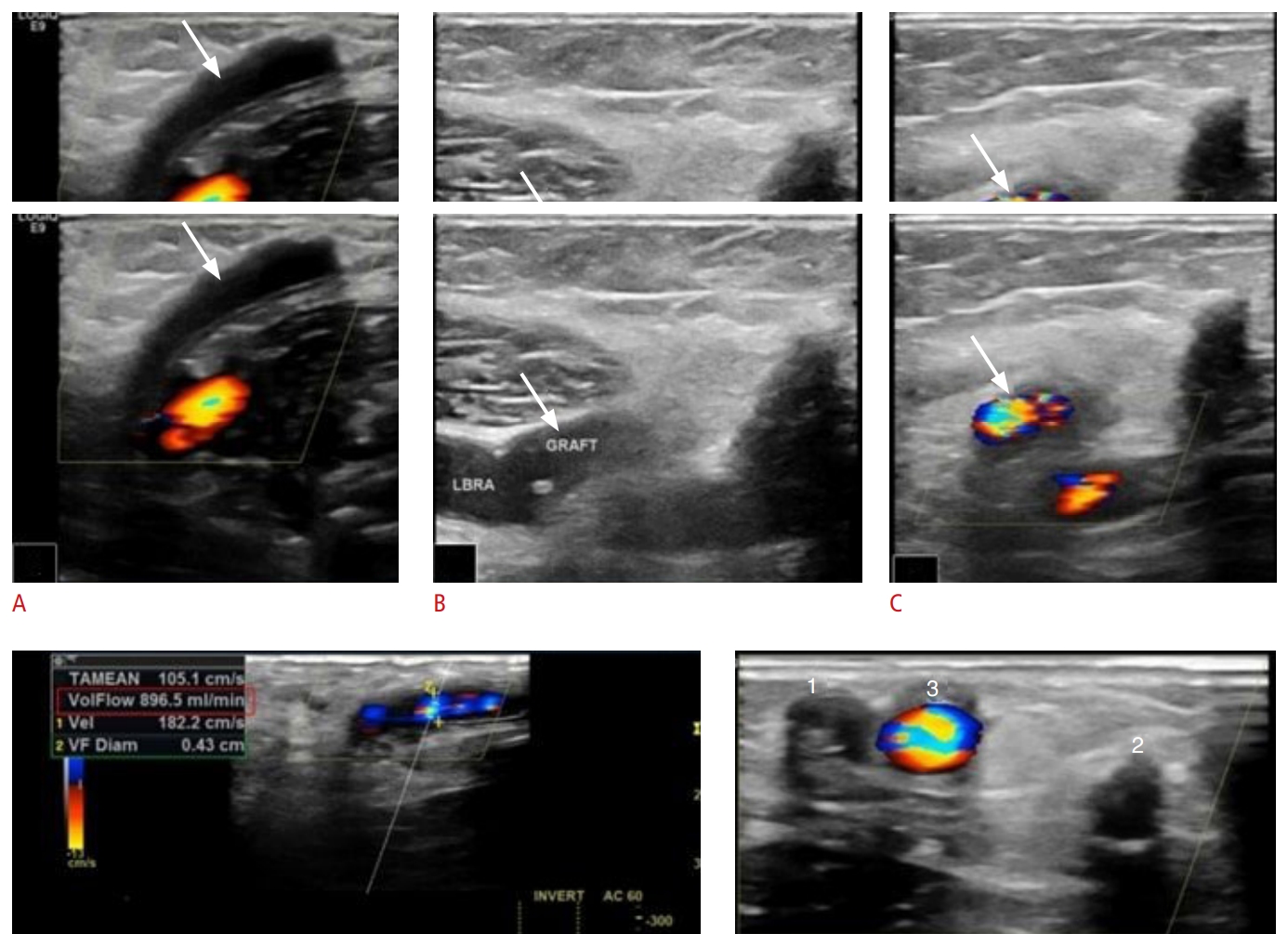 Fig. 9.Thrombosis of arteriovenous graft.The cannulation zone of the arteriovenous graft is patent (A and C, green arrows), as opposed to the cephalic outflow, which is acutely thrombosed (B, red arrow). Consecutive subcutaneous edema is present (D, yellow arrows); subcutaneous edema is typically seen in acute thrombotic disease.
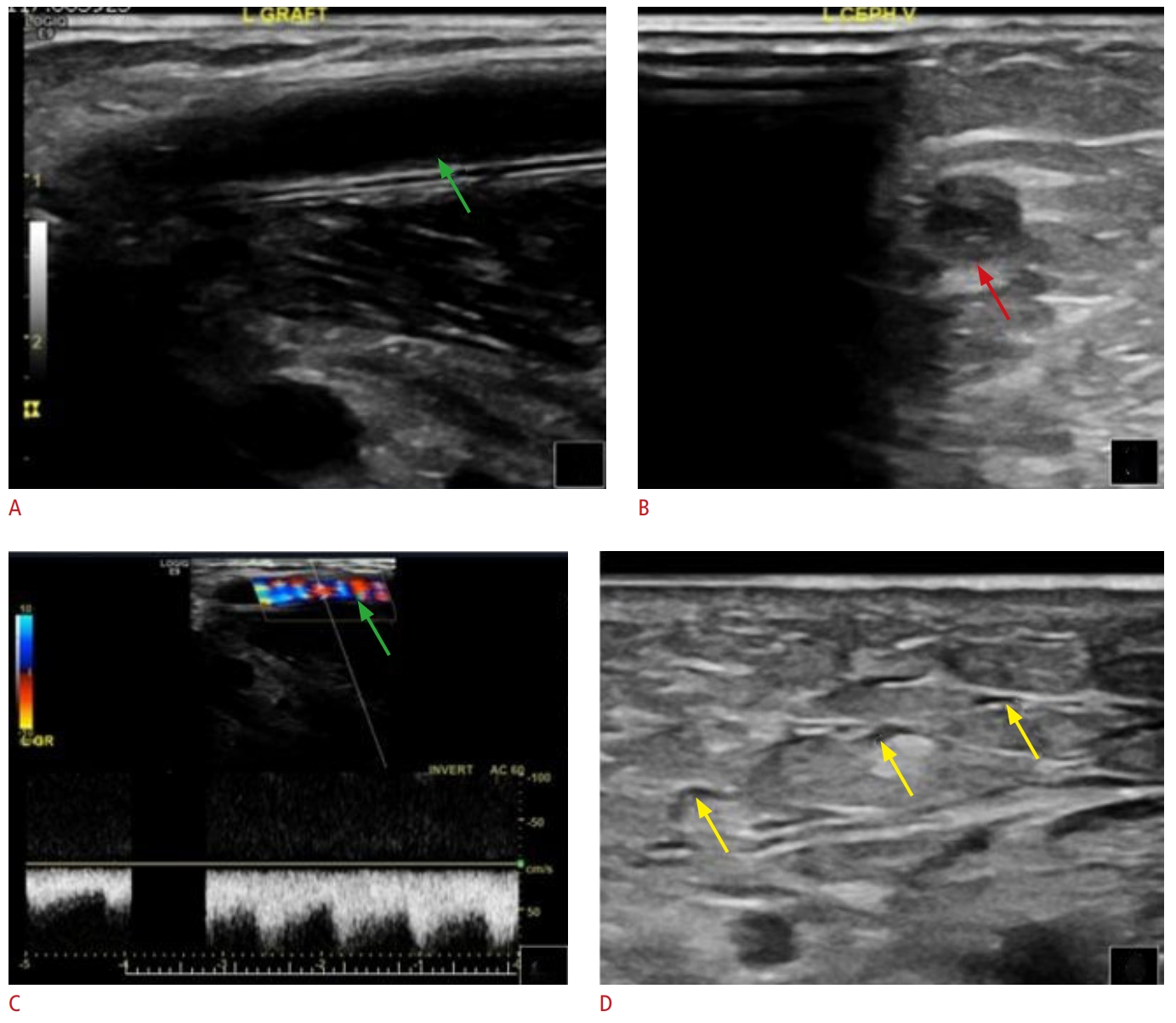 Fig. 10.Thrombosis of arteriovenous graft.In this patient, both upper extremities were accessed at different time points. Chronic cephalic vein thrombosis (A, arrow) and a patent arteriovenous graft (AVG) with adequate blood flow (B; flow volume, 1,431 mL/min; indicated by the red box) are noted on the right side. Graft thrombosis (C and D, white arrows) and subcutaneous edema (C and D, yellow arrows) are visible on the left side. Thus, the left-side access has been abandoned, while the right-side AVG is being used.
 Fig. 11.Iatrogenic hematoma during cannulation.A biconvex area (between calipers) represents iatrogenic hematoma occured during cannulation.
 Fig. 12.Edema (white arrows) due to inflammation adjacent to a patent arteriovenous graft (yellow arrows) on color Doppler ultrasound (A) and B-mode ultrasonography (B).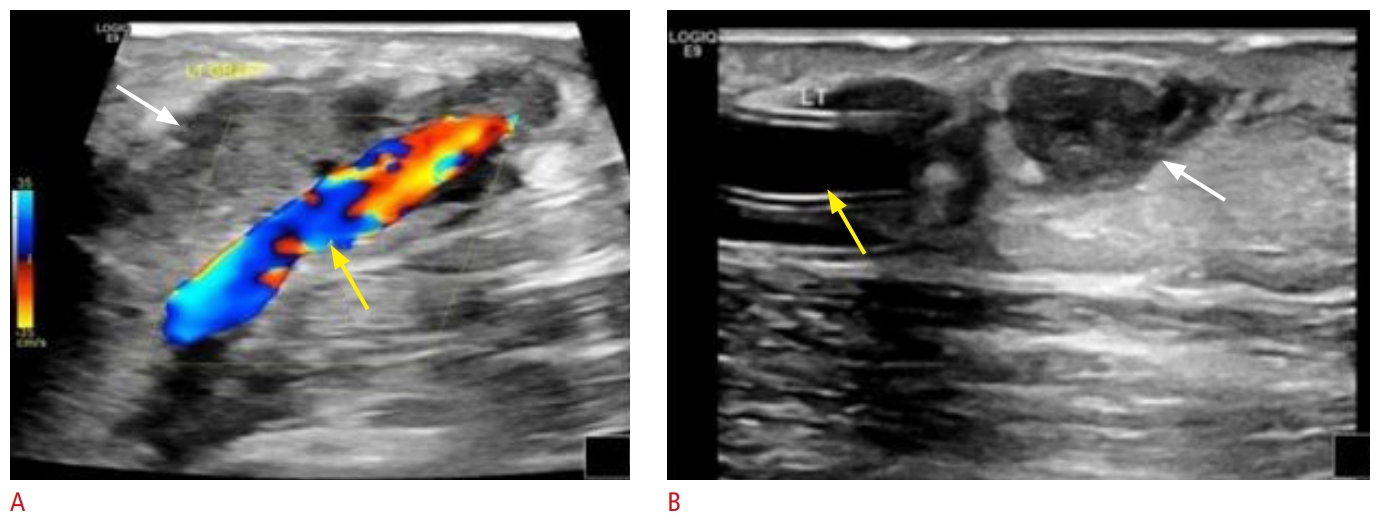 Fig. 13.A 59-year-old man with end-stage renal disease undergoing hemodialysis via a left upper extremity brachiocephalic fistula.The patient presented with a malfunctioning fistula with decreased flow during dialysis. Grayscale, color, and spectral Doppler ultrasound images demonstrated narrowing with turbulent flow (A) and a focal increase in peak systolic velocity (B, C) at the junction of the cephalic arch with the subclavian vein, suggestive of 50% to 99% stenosis. A fistulogram is confirming the presence of focal severe stenosis (blue arrow) (D), which has been treated successfully with plain balloon angioplasty (E, F).
 Fig. 14.A 39-year-old woman with end-stage renal disease undergoing hemodialysis via a right upper extremity brachioaxillary arteriovenous graft.The patient presented with swelling of the right arm. Grayscale (A) as well as color and spectral Doppler (B, C) ultrasound images demonstrate 50% to 99% stenosis of the venous outflow system, with a significant increase in peak systolic velocity just central to the anastomosis. A fistulogram shows the presence of severe venous anastomotic stenosis (blue arrow) (D), and this is being successfully treated with angioplasty and stent-graft placement (E, F).
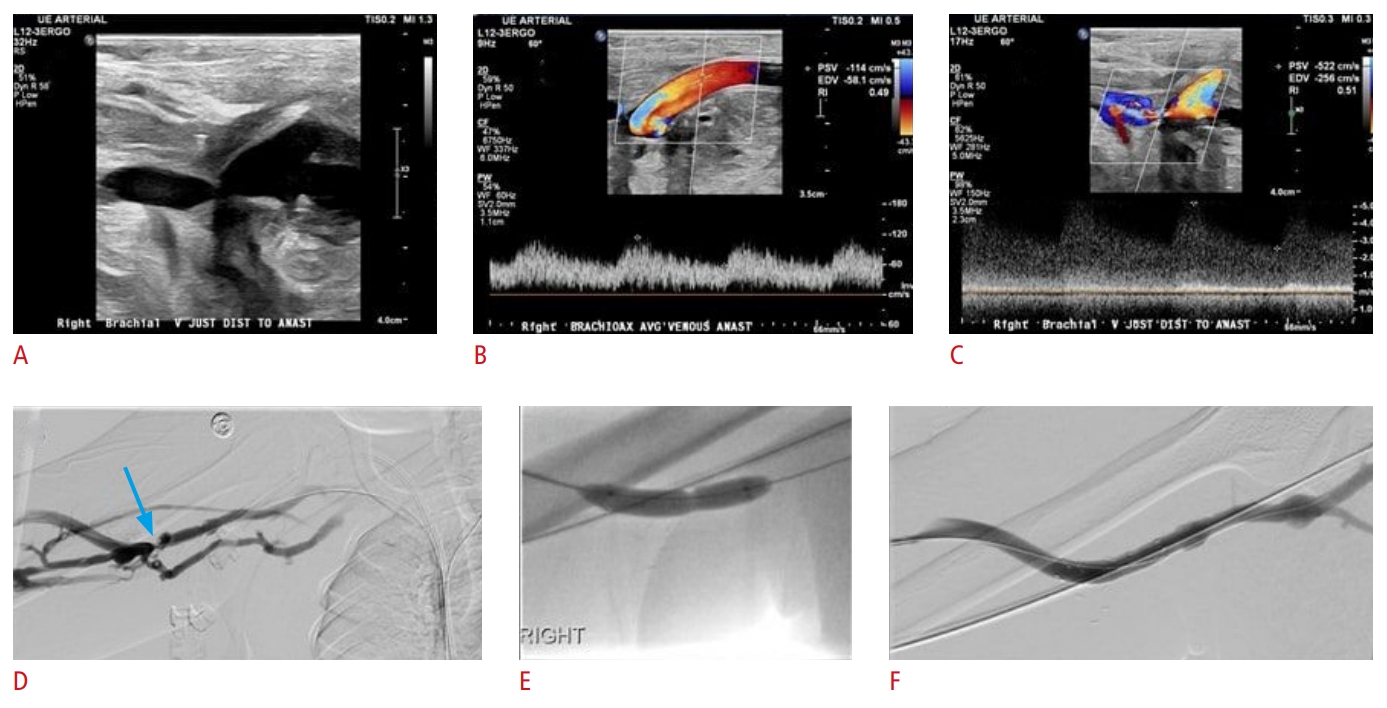 Fig. 15.A 61-year-old man with end-stage renal disease and a malfunctioning left lower extremity femoral-femoral arteriovenous graft (AVG).Fistula palpation demonstrated weak pulse but no thrill. Grayscale, color, and spectral Doppler ultrasound images of the AVG demonstrate focal mid graft stenosis (A-C) as well as focal stenotic plaque at the venous anastomotic site (E-G), resulting in 50% to 99% stenosis. Initial fistulogram findings in accordance with US show the presence of moderate to severe stenosis at the venous anastomosis (yellow arrow) (H) and moderate stenosis of the mid graft (blue arrow) (D). Treatment with balloon angioplasty was pursued in the same procedural session (images not shown).
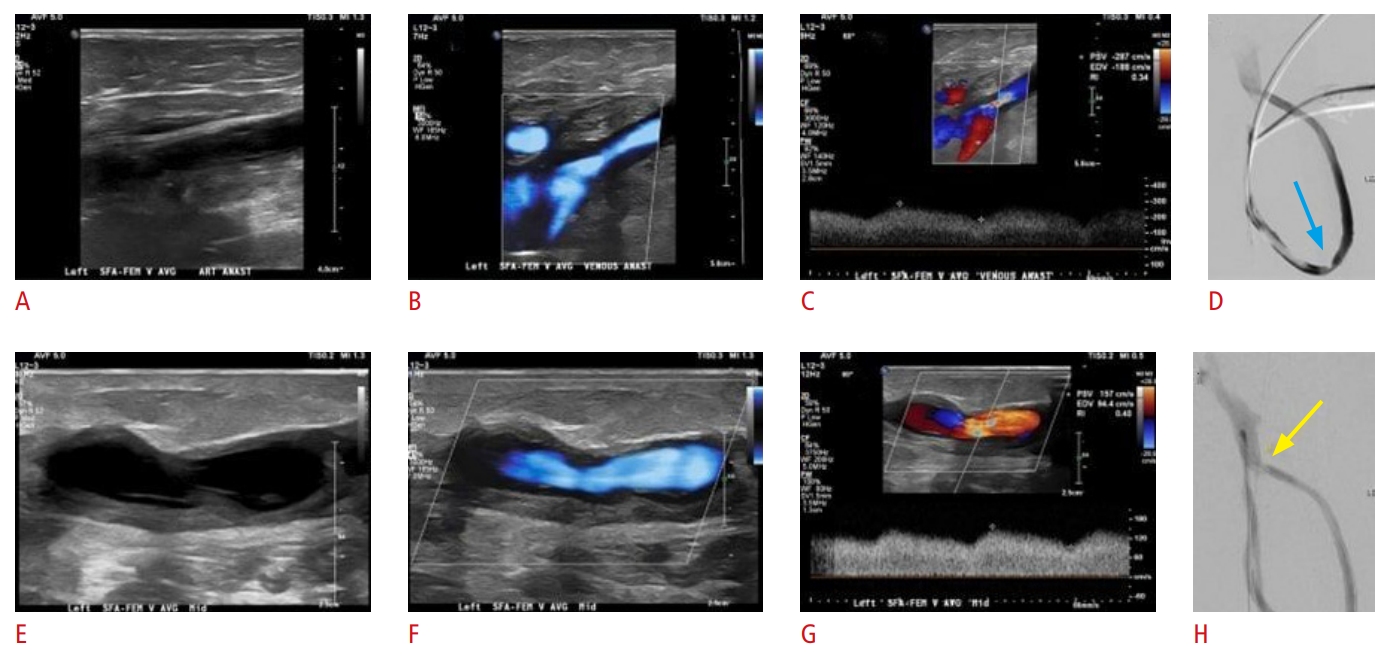 Fig. 16.An 81-year-old man with end-stage renal disease undergoing hemodialysis via a right upper extremity brachiobasilic arteriovenous fistula.The patient presented with declining flow rates during hemodialysis. Color (A, B) and spectral Doppler (C, D) ultrasound images demonstrate turbulent flow and focal significant increase in peak systolic velocity, suggestive of 50% to 99% stenosis in the stented peripheral venous outflow system. A fistulogram is confirming the presence of moderate in-stent stenosis of the venous outflow (blue arrow) (E) and this is being subsequently treated subsequently treated endovascularly.
 Fig. 17.A 70-year-old man with end-stage renal disease undergoing hemodialysis via a left lower extremity femoral-femoral arteriovenous fistula (AVF).The patient presented with high pressures during dialysis. Grayscale and color ultrasound images of the AVF demonstrate 50% to 99% stenosis of the venous outflow (A-C). A fistulogram is further confirming the presence of severe focal stenosis (blue arrow) within the venous outflow tract of the left external iliac vein (D).
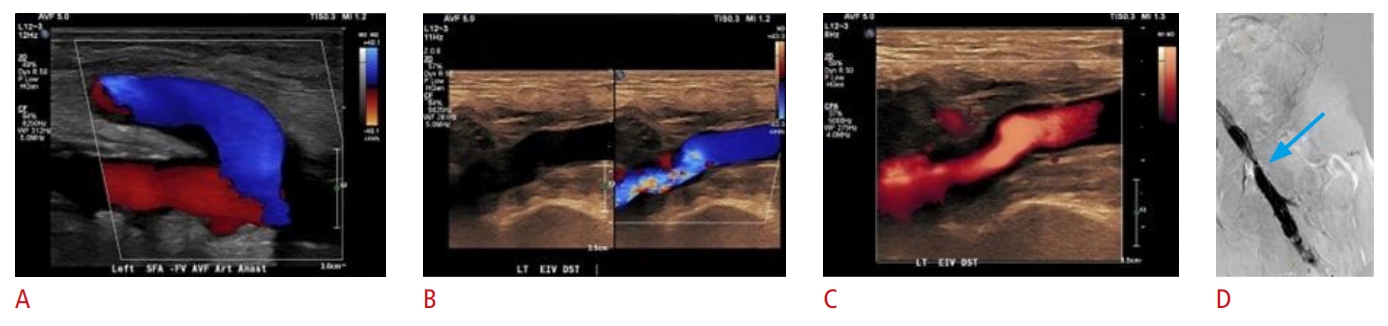 Fig. 18.An arteriovenous fistula (AVF) between the cephalic vein and the brachial artery.The cephalic vein is thrombosed (A, white arrow). The brachial artery is patent (A, yellow arrow), but the blood flow is very low (B, red box). Strain elastography (C) reveals a mixed representation of thrombus stiffness (red and green). This is consistent with an acute on chronic thrombosis of the brachiocephalic AVF.
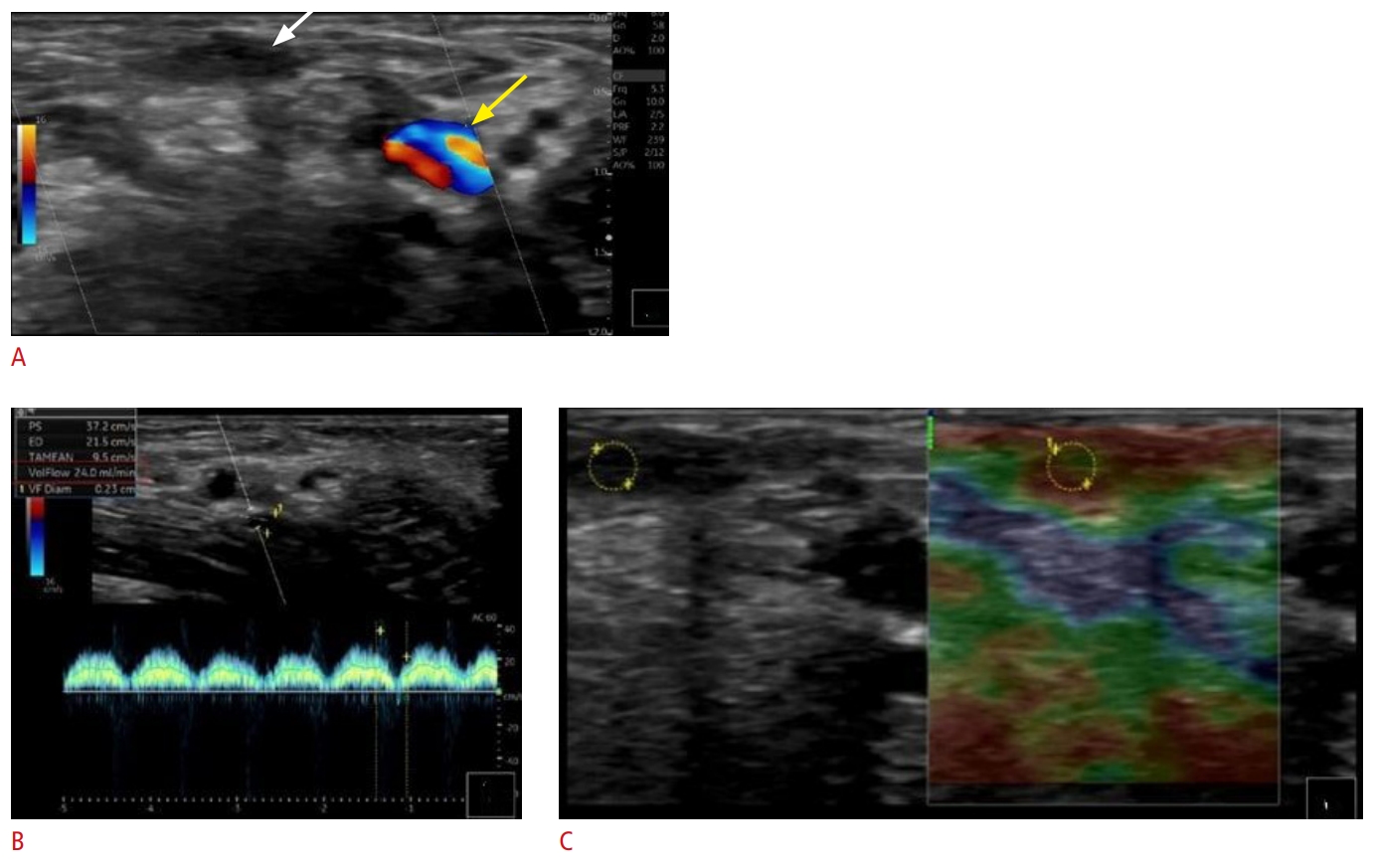 Fig. 19.Table 1.The rule of 6's as a guideline for evaluation of arteriovenous fistula maturation
Table 2.Published US criteria regarding significant stenoses in AVFs and AVGs Table 3.Overview of potential applications of multiparametric US in evaluating upper extremity vascular access
|



 Print
Print facebook
facebook twitter
twitter Linkedin
Linkedin google+
google+
 Download Citation
Download Citation PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI




