AbstractPurposeThis study assessed the performance of transperineal ultrasonography (TPUS) in evaluating the treatment response in children with perianal Crohn’s disease (PACD) compared with pelvic magnetic resonance imaging (MRI).
MethodsThis retrospective study was approved by the Institutional Review Board of our institution, which waived the requirement for informed consent. Twenty-nine patients (19 boys and 10 girls; median age, 14 years [range, 8 to 18 years]) with 56 fistulas were examined. Each fistula’s thickness and abscess size were measured using both modalities, and treatment response was classified as positive or negative based on each modality. The concordance of the classifications was compared between TPUS and pelvic MRI. A receiver operating characteristic curve (ROC) was used to evaluate the performance of TPUS.
ResultsTPUS found 80.4% (45/56) of the fistulas. On MRI, 39 fistulas (70%) were classified as having positive treatment responses, and the remaining 17 as having no response. The agreement of the classifications between TPUS and MRI was moderate (κ=0.486; P<0.001; Spearman ρ=0.573; P<0.001). Based on the ROC analysis with the MRI findings as a reference to distinguish positive from negative treatment responses, TPUS exhibited sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of 63.3%, 93.3%, 95.0%, 56.0%, and 73.3%, respectively.
Perianal Crohn’s disease (PACD) is an inflammatory disease involving the anus, possibly causing fistulas and abscesses in patients with Crohn’s disease [1]. In particular, PACD in children is more likely to manifest as perianal fistulas or abscesses at the time of diagnosis than in adults (9%–15% vs. 5%–9%) [1-6]. Medical treatment using anti-tumor necrosis factor (TNF) agents or immunosuppressants such as methotrexate (MTX) is widely accepted as the initial treatment strategy for PACD, except in cases with overt abscesses [7]. In cases with an insufficient response to the initial medical treatment, further treatment options include escalation of the medication dose, the use of additional medication, or surgical intervention [7]. However, PACD usually has a refractory course, which is closely associated with significant morbidity and severe impairment in quality of life [8,9]. Therefore, frequent and accurate assessments of the treatment response are crucial for the timely optimization of the treatment strategy in the early disease phase, to prevent complications or sequelae from perianal fistulas and improve the outcomes of PACD.
Although examiner-based fistula drainage assessment has been traditionally used as a tool to monitor the treatment response in PACD, this measure might overestimate the treatment response and overlook the residual fistula and subsequent abscess formation when associated with the closure of a cutaneous opening [10]. Therefore, image-based evaluations of PACD activity, including pelvic magnetic resonance imaging (MRI) or endoscopic ultrasonography (EUS), have been widely investigated [11-18]. Among them, the MRI-based scoring systems proposed by Van Assche et al. [11] and Ng et al. [13] are widely used to monitor the treatment response in PACD since pelvic MRI is regarded as the gold-standard method for detecting and measuring perianal fistulas due to its high spatial resolution and diagnostic power.
However, MRI has the disadvantages of being expensive and requiring a long scan time. In particular, when used as a followup imaging modality for Crohn's disease, which requires frequent scans at short intervals, the burden on patients may increase. Furthermore, sedatives are necessary for young children or patients with claustrophobia, and MRI requires gadolinium-based contrast agents, which are associated with a risk of complications, such as brain deposition or nephrotic systemic fibrosis.
Transperineal ultrasonography (TPUS) is a simple, real-time, and practical examination that does not require specific patient preparation. Compared with pelvic MRI, TPUS has the advantages of being inexpensive, noninvasive, widely available, and safe [19-23]. Previous studies found that TPUS might be an appropriate method for detecting perianal fistulas and/or abscesses in children with PACD [21,22]. However, to date, no studies have examined its clinical usefulness compared with that of MRI for monitoring the therapeutic response in PACD. Therefore, the current study aimed to assess the performance of TPUS in evaluating the treatment response in children with PACD compared with pelvic MRI.
This retrospective study was approved by the Institutional Review Board of Seoul National University Bundang Hospital, which waived the requirement for informed consent (IRB No. B-2105-687-102).
Between January 2013 and February 2020, 125 patients aged <19 years with Crohn's disease who underwent TPUS or pelvic MRI at the authors’ affiliated hospital were consecutively enrolled in this study. Among them, 56 were selected based on the availability of two or more pelvic MRI scans and TPUS examination reports. Twenty-seven patients were removed from consideration according to the following exclusion criteria: (1) a >14-day interval between each MRI and TPUS examination (n=22), (2) absence of perianal lesions (n=2), and (3) a >2-year interval between baseline and follow-up examinations (n=3). Finally, 29 patients were included in the analysis. The demographic data and information related to the treatment for PACD were collected from the electronic medical records.
TPUS was performed without preparations such as enema administration or premedication by two experienced pediatric radiologists (J.Y.K. and Y.J.R. with 16 and 10 years of clinical experience, respectively). The patients were positioned in the left lateral decubitus and knee-chest position. A 5–12-MHz linear high-frequency transducer (iU22 or EPIQ 7, Philips Medical Systems, Andover, MA, USA) was wrapped in a latex cover after applying a contact gel on the probe surface. A large amount of gel was also used on the surface of the latex cover to minimize pain and enhance the perianal lesion visibility. TPUS was performed on the sagittal plane; if possible, ultrasonography (US) images on the coronal plane and along the fistula’s long axis were also obtained. The normal anatomy of the anal canal and perianal region of TPUS is shown in Fig. 1. Perianal disease locations were annotated on US images according to the anal clock face [24]. If the location of the fistula skin opening and anal canal opening differed by >60° (2 o’clock), each location was described on the TPUS reading.
Two radiologists (Y.J.R. and J.H.J. with 10 and 3 years of clinical experience), respectively blinded to the MRI results, retrospectively reviewed the baseline and follow-up TPUS images. The radiologists independently measured the maximum thickness of the perianal fistula and/or the maximum size of the perianal abscess in the same plane at baseline and follow-up TPUS.
All pelvic MRI scans were obtained using a 3.0 T MRI apparatus (Ingenia, Philips Medical Systems, Amsterdam, Netherlands). The imaging protocol included axial, coronal, and sagittal T2-weighted images (T2WI) acquired with a turbo spin-echo (TSE) fat-suppressed spectral attenuated inversion-recovery sequence (repetition time [TR]/echo time [TE], 3,660–4,542/70 ms; section thickness, 3 mm; field of view [FOV], 200×200–250×250 mm; matrix, 328×326; flip angle [FA], 90°; one acquired signal; echo train length [ETL], 18). After intravenous contrast injection, axial T1-weighted images were acquired with a TSE spectral pre-saturation inversion-recovery sequence (TR/TE, 515–629/10 ms; section thickness, 3 mm; FOV, 230×230 mm; matrix, 328×326; FA, 90°; one acquired signal; ETL, 7). The dose of contrast agent used (Gadobutrol, Gadovist, Bayer Schering Pharma, Berlin, Germany) was 0.1 mL/kg.
The baseline and follow-up pelvic MRI scans were reviewed retrospectively and interpreted by two radiologists with 10 and 3 years of clinical experience, blinded to the TPUS results. The location of the perianal lesions was determined according to the anal clock face. The radiologists independently measured the maximum thickness of the perianal fistula on the axial plane of the T2WI of each MRI scan. Moreover, if an abscess (defined as a fluid-filled cavity with a diameter >3 mm [11]) accompanied a fistula, it was evaluated on axial, coronal, and sagittal T2WI, and the maximum diameter was recorded.
A radiologist (Y.J.R. with 10 years of clinical experience) also assessed classifications of the perianal fistulas according to the Parks criteria, the St. James’s University Hospital system, and American Gastroenterological Association (AGA) classification. The Parks classification describes four types of perianal fistulas: intersphincteric, transsphincteric, suprasphincteric, and extrasphincteric (Fig. 2). The St. James's Hospital classification system consists of five grades: grade 1, simple linear intersphincteric fistula; grade 2, intersphincteric with abscess or secondary tract; grade 3, transsphincteric; grade 4, transsphincteric fistula with an abscess or secondary fistulous tract within the ischiorectal or ischioanal fossa; grade 5, supralevator and translevator. A "superficial (submucosal) fistula" is defined as a fistula that occurs in the low anal canal and extends to the skin surface without involving the internal or external anal sphincters [25-27]. Although the Parks and St. James’s original classifications do not include superficial fistulas, they are currently commonly encountered in clinical practice, so we added superficial fistulas to both classifications as part of the criteria for this study [25].
In addition, fistulas in the AGA classification system are divided into either simple or complex fistulas. Single superficial, intersphincteric, and low transsphincteric (below the dentate line) fistulas without abscesses, rectovaginal fistulas, or anorectal stricture are called simple fistulas; high transsphincteric, extrasphincteric, and suprasphincteric fistulas, as well as fistulas with secondary tracts, abscesses, horseshoeing, rectovaginal fistulas, or anorectal stricture are called complex fistulas.
Two radiologists (Y.J.R. and J.H.J.) assessed and classified by consensus the treatment response of each perianal fistula into four categories based on the MRI and TPUS findings. The predetermined per-person MRI classification proposed by Ng et al. [12,13] was modified to develop a per-lesion assessment. For the MRI and TPUS classification, the treatment response was considered complete remission (CR) when the lesion disappeared, partial remission (PR) when the fistula thickness or abscess size decreased by >50%; and aggravated disease (AD) when a new perianal lesion appeared or the fistula thickness or abscess size increased by >50%; the remaining cases were classified as stable disease (SD). The four groups mentioned above were united into two larger groups: positive (CR, PR) and negative response (SD, AD). The concordance of treatment response evaluation between MRI and TPUS was examined. The location of the perianal lesions was assessed based on the anal clock face for both imaging modalities, which were considered to correspond if the lesions were reported to be within 30° (1 o’clock) in different examinations. If the locations of a perianal fistula and abscess coincided, they were considered a single lesion (fistula accompanied by an abscess). If the evaluation of the treatment response for fistulas and abscesses at the same location differed, the abscess evaluation was included in the analysis.
Concordance between TPUS and MRI in the treatment response evaluation was assessed using the Cohen kappa and Spearman rank correlation tests. Receiver operating characteristic (ROC) curve analysis was used to assess the performance of TPUS based on pelvic MRI findings. The MRI findings were compared between TPUS-detected and undetected lesions and between TPUS-MRI concordant and discordant lesions, including the classification (superficial vs. non-superficial), the AGA classification type (simple vs. complex), fistular thickness, number of fistulas per patient, abscess (presence vs. absence) and abscess size. Categorical variables were evaluated using the Pearson chi-square or Fisher exact test, while the continuous variables were evaluated with the Mann-Whitney U test. Interobserver agreement for perianal fistula thickness was evaluated by calculating the intraclass correlation coefficient (ICC) and the intraobserver agreement between TPUS and MRI evaluations with Pearson correlation coefficients.
Kappa and ICC values <0.2 were regarded as slight agreement, 0.2–0.4 as fair, 0.4–0.6 as moderate, 0.6–0.8 as substantial, and >0.8 as almost perfect agreement, according to the Fleiss grading system [28]. Spearman and Pearson correlation coefficients <0.2 were regarded as weak to no correlations, 0.2–0.4 as weak, 0.4–0.6 as moderate, 0.6–0.8 as strong, and 0.8–1.0 as very strong correlations [29]. For all statistical analyses, a two-sided P-value <0.05 was considered to indicate statistical significance. All statistical analyses were performed using PASW Statistics (version 22.0, IBM Corp., Armonk, NY, USA) and MedCalc software (MedCalc 20.019, MedCalc Software, Ostend, Belgium).
In total, 29 patients with PACD were enrolled (19 boys and 10 girls; median age, 14 years [range, 8 to 18 years]). There were 13 patients with one fistula, nine patients with two fistulas, five patients with three fistulas, one patient with four fistulas, and one patient with six fistulas. Four patients had only PACD without small bowel or colon involvement; one underwent surgery (fistulectomy) for PACD; 14 received immunosuppressants, including steroids and MTX; 14 were treated with an anti-TNF agent, such as infliximab, in addition to steroids and MTX. Table 1 shows the demographic and clinical data of the study population.
Fifty-six fistulas were detected on pelvic MRI, and most perianal fistulas (73%, 41/56) were intersphincteric. Fourteen abscesses were detected on baseline MRI and two additional abscesses were found on follow-up MRI. TPUS found 80.4% (45/56) of the fistulas. Moreover, 37 fistulas were classified as simple and 19 as complex, based on the AGA classification. Table 2 shows the numbers of perianal fistulas detected on pelvic MRI according to the Parks and St. James’s classifications.
MRI revealed that 39 fistulas (70%) showed a positive treatment response (CR, n=20; PR, n=19), and the remaining 17 fistulas had a negative response (SD, n=11; AD, n=6). Based on the ROC analysis with the MRI findings as a reference to distinguish positive from negative treatment responses, TPUS exhibited a sensitivity, specificity, positive predictive value (PPV), negative predictive value, and accuracy of 63.3%, 93.3%, 95.0%, 56.0%, and 73.3%, respectively. Table 3 summarizes the results of ROC analyses for TPUS. The concordance between TPUS and MRI in the evaluation of treatment response was moderate (κ=0.486; P<0.001; Spearman ρ=0.573; P<0.001) (Figs. 3, 4).
The treatment response evaluation was discordant for 12 of the 45 lesions detected on both TPUS and pelvic MRI. Among these, 10 (83.3%) lesions were classified as SD on TPUS, while they were considered CR or PR on MRI (Table 4, Figs. 5, 6). Of the other two discordant lesions, one lesion was classified as AD on TPUS and PR on pelvic MRI. On follow-up MRI, the fistula showed AD, and the accompanying abscess disappeared; however, only the fistula showing AD was detected on baseline and follow-up TPUS. For the other lesion, TPUS yielded an assessment of CR and MRI resulted in a judgment of SD. The fistula disappeared on follow-up TPUS; however, the lesion persisted on follow-up MRI. Nonetheless, there were no significant differences in MRI findings between TPUS-MRI concordant and discordant groups in terms of classification (Parks and AGA), fistula thickness, number of fistulas per patient presence of abscesses, and abscess size (Supplementary Table 1).
Comparing the baseline MRI findings between the fistulas detected and undetected using TPUS, superficial fistulas were more frequent in the undetected group than in the detected group (P=0.005) (Fig. 6). The fistula thickness measured on MRI in the detected group was significantly higher than that in the undetected group (P=0.009) (Fig. 6). No significant differences were observed between the two groups in the AGA classification, number of fistulas per patient, presence of abscesses, and abscess size. These results are summarized in Table 5.
The interobserver agreement for the measurements of the fistula thickness was excellent (ICC, 0.890 to 0.953; P<0.001) (Supplementary Table 2). Furthermore, the intraobserver agreement for thickness measurement based on TPUS and MRI findings showed a moderate positive correlation (Pearson correlation coefficient, 0.432 to 0.484; P<0.001) (Supplementary Table 3).
To the authors’ knowledge, this study presents the first comparison between TPUS and pelvic MRI to evaluate the treatment response in PACD. This study revealed moderate agreement between TPUS and MRI in the evaluation of treatment response, and based on MRI as a reference, TPUS demonstrated excellent specificity and PPV in detecting a positive treatment response. Thus, TPUS is an appropriate method to evaluate the therapeutic response of PACD in children, and it can be a useful adjuvant imaging modality for pelvic MRI to monitor the treatment response of PACD in children when initial TPUS detects PACD with a location and imaging features comparable to those visualized on MRI.
Given its excellent specificity and PPV for detecting a positive treatment response, TPUS can be especially useful for the timely selection of patients who require further imaging, such as pelvic MRI, to confirm non-responsiveness. Although pelvic MRI is considered superior to other imaging modalities for monitoring the therapeutic response in PACD [11-17], TPUS is more economical, less restricted by place or equipment, and has a lower requirement for sedative agents than pelvic MRI, making it more feasible for frequent and repeated use. Thus, for non-responders, frequent monitoring of short intervals using TPUS can help guide early follow-up MRI scans and timely treatment change, including therapy escalation and surgical intervention.
The relatively low sensitivity of TPUS for detecting a positive treatment response compared to the specificity can be explained by fibrous changes in the fistula after remission in the chronic phase, resulting in no change in the fistula thickness measured on TPUS (Figs. 4–6) [30]. Therefore, TPUS might be especially useful in evaluating the treatment response of PACD in early induction therapy, before overt fibrous changes develop in the lesion.
Approximately 20% of fistulas were not detected using TPUS. The TPUS-undetected fistulas were thinner and located more superficially than the TPUS-detected fistulas. Superficial perianal fistulas are difficult to distinguish from the typical multi-layered anal walls because, on TPUS, the fistula appears as focal hypoechoic thickening of the anal wall (Fig. 4). Furthermore, thin lesions are challenging to detect with TPUS because the difference in echogenicity is less pronounced than the signal intensity difference on MRI. Therefore, the evaluation accuracy using TPUS might be limited for fistulas with these characteristics, whereas thick and intersphincteric or transsphincteric lesions can be adequately evaluated. Consequently, the most appropriate follow-up test modality can be selected based on the lesion’s characteristics.
Interestingly, the intraobserver agreement on fistula thickness between TPUS and MRI measurements was moderate. This finding could be due to the different planes used for thickness measurement between TPUS and MRI; moreover, the edema surrounding the fistula was included on MRI and excluded on TPUS. Therefore, when TPUS and pelvic MRI are used alternatively for PACD evaluation, the treatment response should be assessed with appropriate recognition of the possibility that there may be differences in fistula thickness between the two modalities.
This study has some limitations arising from its retrospective design. First, the PACD activity classification used herein was not validated; instead, the predefined MRI score proposed by Ng et al. [12,13] was modified and applied owing to the lack of consensus on evaluating the treatment response using TPUS. Further studies are needed to validate this scoring system. Second, the clinical response was not evaluated using the Fistula Drainage Assessment or Perianal Disease Activity Index (PDAI) score. Since we focused on evaluating whether TPUS could replace MRI, we assessed only the radiological response evaluation. Furthermore, per-lesion rather than per-person analyses were performed; thus, the findings cannot be interpreted in terms of the individual clinical response. Additionally, the PDAI scoring system is unfeasible to apply to children because it includes the restriction of sexual activity as a parameter. Third, the sample size was relatively small. A further prospective study that includes a larger population is warranted to strengthen the statistical power. Fourth, the performance of EUS could not be assessed because EUS is not performed in children at the authors’ institution except when necessary for preoperative evaluation.
In conclusion, TPUS can be an appropriate adjuvant imaging modality for pelvic MRI to evaluate the treatment response of PACD in children when initial TPUS detects PACD with a location and imaging features comparable to those visualized on MRI. In addition, TPUS might be useful for monitoring the therapeutic response of PACD at short intervals in conjunction with a physical examination, especially at the beginning of induction therapy.
NotesAuthor Contributions Conceptualization: Ryu YJ, Kim JY. Data acquisition: Jung JH, Ryu YJ, Kim JY, Yang HR. Data analysis or interpretation: Jung JH, Ryu YJ. Drafting of the manuscript: Jung JH, Ryu YJ. Critical revision of the manuscript: Ryu YJ, Kim JY, Yang HR. Approval of the final version of the manuscript: all authors. AcknowledgementsThis work was supported by grant no. 02-2021-0030 from the Seoul National University Bundang Hospital Research fund.
Supplementary MaterialSupplementary Table 1.Comparison of MRI findings between concordant and discordant cases between TPUS and MRI (https://doi.org/10.14366/usg.22057).
Supplementary Table 2.Interobserver agreement of fistular thickness (https://doi.org/10.14366/usg.22057).
Supplementary Table 3.Intraobserver correlation of measurement of fistular thickness between TPUS and MRI (https://doi.org/10.14366/usg.22057).
References1. de Zoeten EF, Pasternak BA, Mattei P, Kramer RE, Kader HA. Diagnosis and treatment of perianal Crohn disease: NASPGHAN clinical report and consensus statement. J Pediatr Gastroenterol Nutr 2013;57:401–412.
2. Schwartz DA, Loftus EV Jr, Tremaine WJ, Panaccione R, Harmsen WS, Zinsmeister AR, et al. The natural history of fistulizing Crohn's disease in Olmsted County, Minnesota. Gastroenterology 2002;122:875–880.
3. Singer AA, Gadepalli SK, Eder SJ, Adler J. Fistulizing Crohn's disease presenting after surgery on a perianal lesion. Pediatrics 2016;137:e20152878.
4. Keljo DJ, Markowitz J, Langton C, Lerer T, Bousvaros A, Carvalho R, et al. Course and treatment of perianal disease in children newly diagnosed with Crohn's disease. Inflamm Bowel Dis 2009;15:383–387.
5. Vernier-Massouille G, Balde M, Salleron J, Turck D, Dupas JL, Mouterde O, et al. Natural history of pediatric Crohn's disease: a population-based cohort study. Gastroenterology 2008;135:1106–1113.
6. Park JJ, Yang SK, Ye BD, Kim JW, Park DI, Yoon H, et al. Second Korean guidelines for the management of Crohn's disease. Korean J Gastroenterol 2017;69:29–54.
7. Steinhart AH, Panaccione R, Targownik L, Bressler B, Khanna R, Marshall JK, et al. Clinical practice guideline for the medical management of perianal fistulizing Crohn's disease: the Toronto Consensus. Inflamm Bowel Dis 2019;25:1–13.
8. Wise PE, Schwartz DA. Management of perianal Crohn's disease. Clin Gastroenterol Hepatol 2006;4:426–430.
9. Maconi G, Ardizzone S, Greco S, Radice E, Bezzio C, Bianchi Porro G. Transperineal ultrasound in the detection of perianal and rectovaginal fistulae in Crohn's disease. Am J Gastroenterol 2007;102:2214–2219.
10. Present DH, Rutgeerts P, Targan S, Hanauer SB, Mayer L, van Hogezand RA, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med 1999;340:1398–1405.
11. Van Assche G, Vanbeckevoort D, Bielen D, Coremans G, Aerden I, Noman M, et al. Magnetic resonance imaging of the effects of infliximab on perianal fistulizing Crohn's disease. Am J Gastroenterol 2003;98:332–339.
12. Ng SC, Plamondon S, Gupta A, Burling D, Kamm MA. Prospective assessment of the effect on quality of life of anti-tumour necrosis factor therapy for perineal Crohn's fistulas. Aliment Pharmacol Ther 2009;30:757–766.
13. Ng SC, Plamondon S, Gupta A, Burling D, Swatton A, Vaizey CJ, et al. Prospective evaluation of anti-tumor necrosis factor therapy guided by magnetic resonance imaging for Crohn's perineal fistulas. Am J Gastroenterol 2009;104:2973–2986.
14. Tougeron D, Savoye G, Savoye-Collet C, Koning E, Michot F, Lerebours E. Predicting factors of fistula healing and clinical remission after infliximab-based combined therapy for perianal fistulizing Crohn's disease. Dig Dis Sci 2009;54:1746–1752.
15. Karmiris K, Bielen D, Vanbeckevoort D, Vermeire S, Coremans G, Rutgeerts P, et al. Long-term monitoring of infliximab therapy for perianal fistulizing Crohn's disease by using magnetic resonance imaging. Clin Gastroenterol Hepatol 2011;9:130–136.
16. Tozer P, Ng SC, Siddiqui MR, Plamondon S, Burling D, Gupta A, et al. Long-term MRI-guided combined anti-TNF-alpha and thiopurine therapy for Crohn's perianal fistulas. Inflamm Bowel Dis 2012;18:1825–1834.
17. Yan X, Zhu M, Feng Q, Yan Y, Peng J, Xu X, et al. Evaluating the effectiveness of infliximab on perianal fistulizing Crohn's disease by magnetic resonance imaging. Gastroenterol Rep (Oxf) 2019;7:50–56.
18. Wiese DM, Beaulieu D, Slaughter JC, Horst S, Wagnon J, Duley C, et al. Use of endoscopic ultrasound to guide adalimumab treatment in perianal Crohn's disease results in faster fistula healing. Inflamm Bowel Dis 2015;21:1594–1599.
19. Maconi G, Tonolini M, Monteleone M, Bezzio C, Furfaro F, Villa C, et al. Transperineal perineal ultrasound versus magnetic resonance imaging in the assessment of perianal Crohn's disease. Inflamm Bowel Dis 2013;19:2737–2743.
20. Wedemeyer J, Kirchhoff T, Sellge G, Bachmann O, Lotz J, Galanski M, et al. Transcutaneous perianal sonography: a sensitive method for the detection of perianal inflammatory lesions in Crohn's disease. World J Gastroenterol 2004;10:2859–2863.
21. Lee EH, Yang HR, Kim JY. Comparison of transperianal ultrasound with colonoscopy and magnetic resonance imaging in perianal Crohn disease. J Pediatr Gastroenterol Nutr 2018;66:614–619.
22. Hwang JY, Yoon HK, Kim WK, Cho YA, Lee JS, Yoon CH, et al. Transperineal ultrasonography for evaluation of the perianal fistula and abscess in pediatric Crohn disease: preliminary study. Ultrasonography 2014;33:184–190.
23. Bor R, Farkas K, Balint A, Szucs M, Abraham S, Milassin A, et al. Prospective comparison of magnetic resonance imaging, transrectal and transperineal sonography, and surgical findings in complicated perianal Crohn disease. J Ultrasound Med 2016;35:2367–2372.
24. Nevler A, Beer-Gabel M, Lebedyev A, Soffer A, Gutman M, Carter D, et al. Transperineal ultrasonography in perianal Crohn's disease and recurrent cryptogenic fistula-in-ano. Colorectal Dis 2013;15:1011–1018.
25. Ziech M, Felt-Bersma R, Stoker J. Imaging of perianal fistulas. Clin Gastroenterol Hepatol 2009;7:1037–1045.
26. O'Malley RB, Al-Hawary MM, Kaza RK, Wasnik AP, Liu PS, Hussain HK. Rectal imaging: part 2, Perianal fistula evaluation on pelvic MRI: what the radiologist needs to know. AJR Am J Roentgenol 2012;199:W43–W53.
27. Sheedy SP, Bruining DH, Dozois EJ, Faubion WA, Fletcher JG. MR imaging of perianal Crohn disease. Radiology 2017;282:628–645.
28. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–174.
Normal anal canal anatomy on transperineal ultrasonography.A, B. Mid-sagittal and mid-coronal transperineal ultrasonographic images of the anal canal show a hypoechoic, internal anal sphincter (arrowhead) and a hyperechoic, external anal sphincter (arrow) on both sides of the anal canal (C). An ischioanal fossa (IAF) is located lateral and posterior to the external anal sphincter.
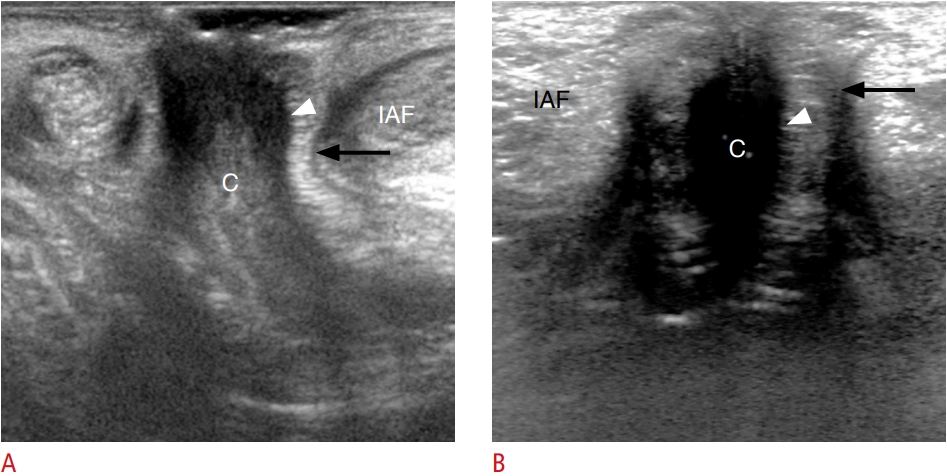 Fig. 1.A 14-year-old boy with a positive treatment response (complete remission) on both transperineal ultrasonography (TPUS) and magnetic resonance imaging (MRI).A, C. Sagittal TPUS and sagittal fatsuppressed T2-weighted images show a perianal fistula at the 12-o’clock location (arrow). B, D. On follow-up TPUS and MRI, the perianal fistula completely disappeared after steroid and methotrexate treatment.
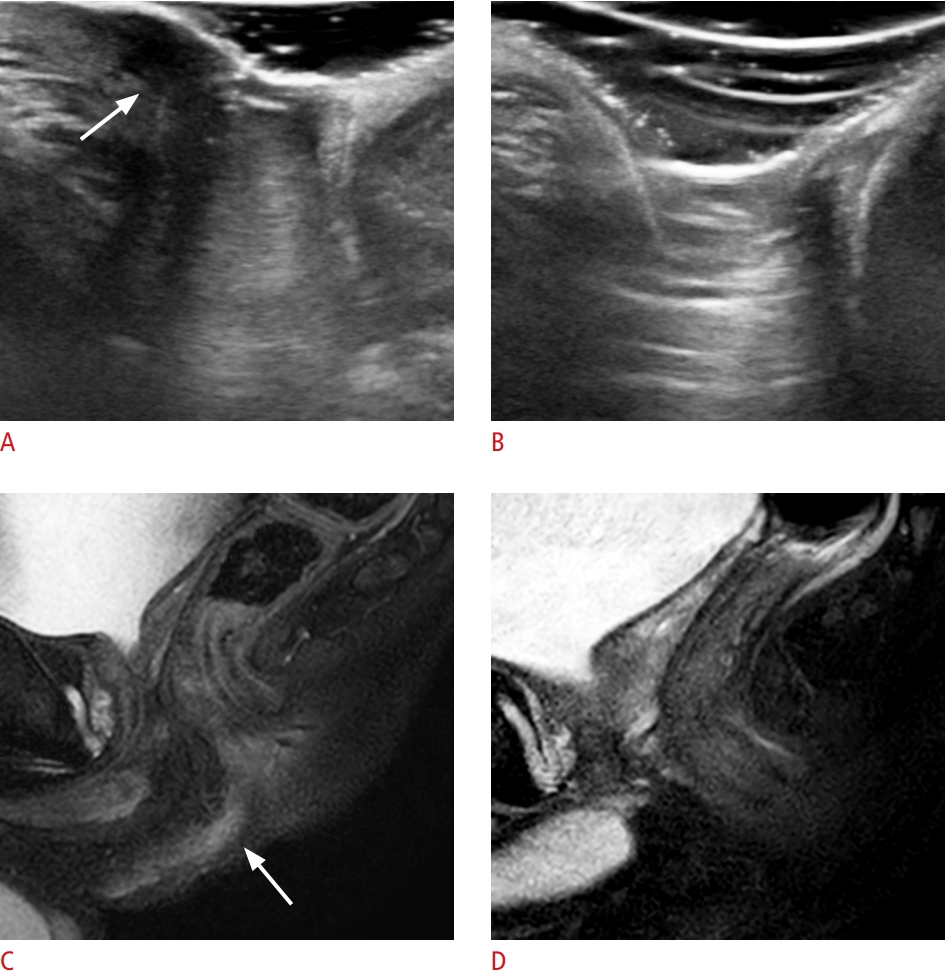 Fig. 3.A 17-year-old boy with a positive treatment response (partial remission) on both transperineal ultrasonography (TPUS) and magnetic resonance imaging (MRI).A, C. Sagittal TPUS and sagittal fatsuppressed T2-weighted images demonstrate a perianal fistula (arrow) and abscess (arrowhead) at the 12-o’clock location. B, D. Follow-up TPUS and MRI reveal partial remission of the perianal fistula (arrow) and abscess (arrowhead) after infliximab, steroid, and methotrexate treatment.
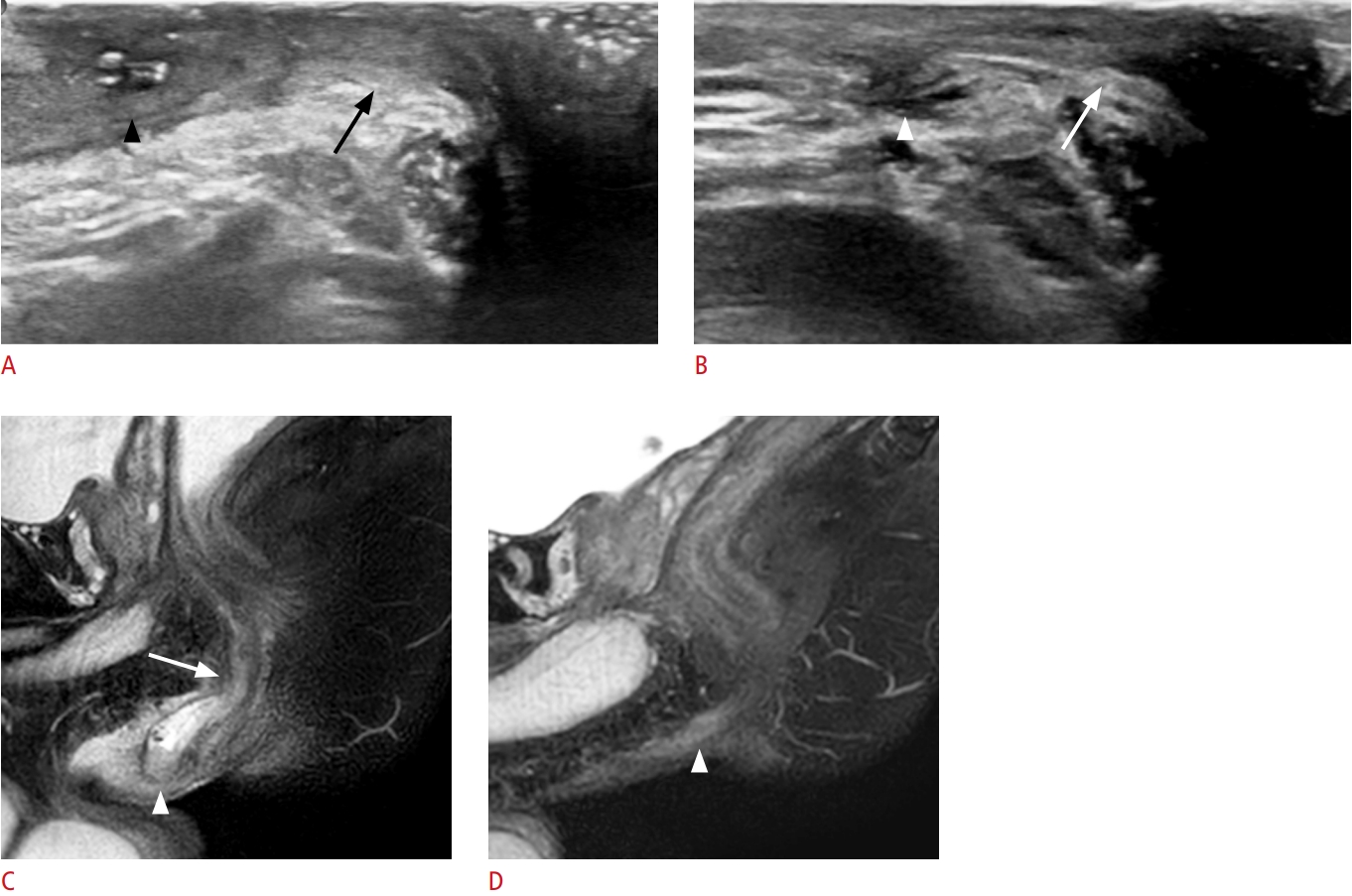 Fig. 4.A 12-year-old boy with discordant treatment response evaluations between transperineal ultrasonography (TPUS) and magnetic resonance imaging.A, C, E. On baseline sagittal TPUS, sagittal and coronal fat-suppressed T2-weighted images, a perianal fistula (arrow) is noted at the 3-o’clock location. B. On follow-up TPUS after infliximab, steroid, and methotrexate treatment, the perianal fistula (arrow) appears to persist. D, F. Follow-up sagittal and coronal fat-suppressed T2-weighted images show complete remission of the perianal fistula (arrow).
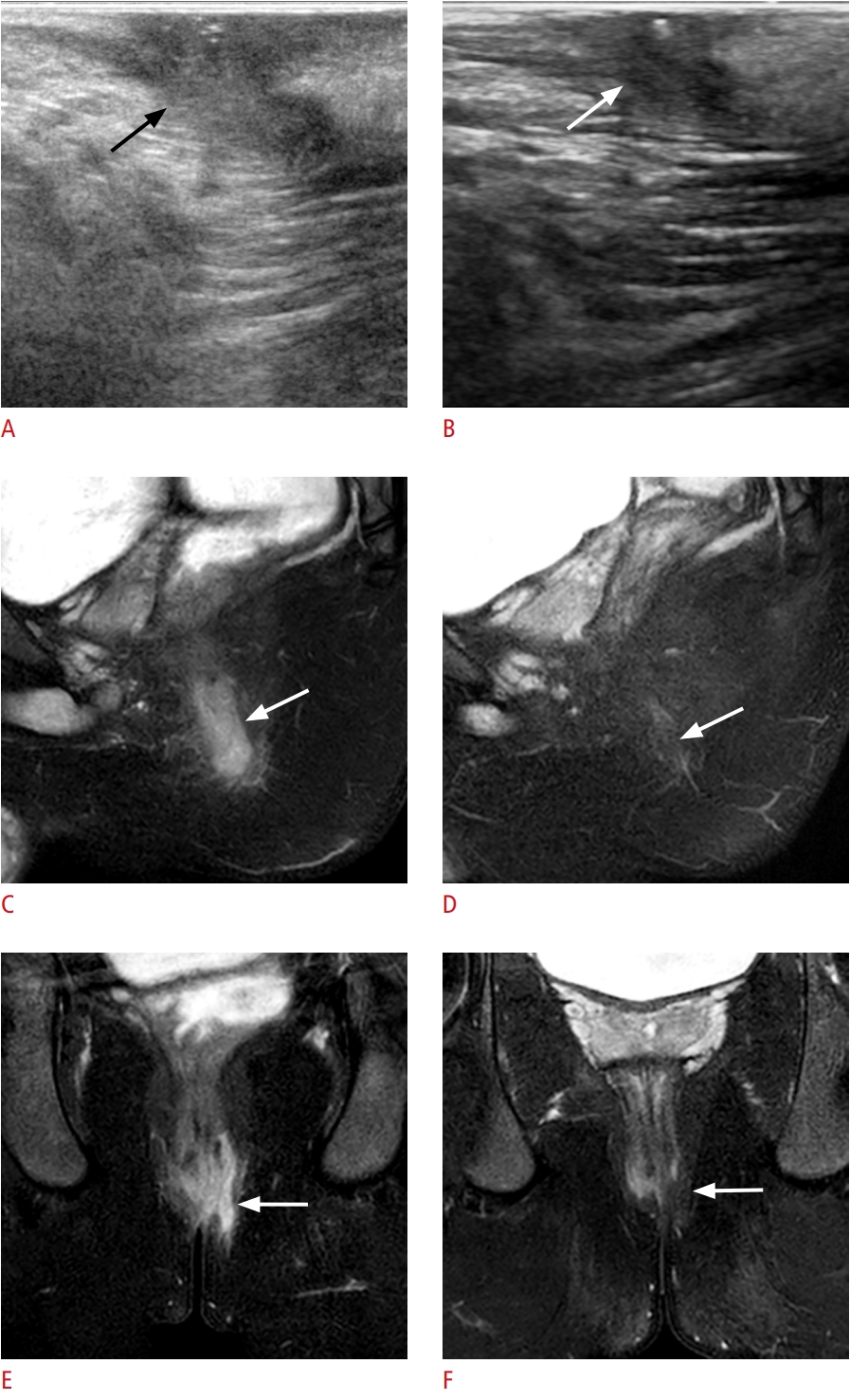 Fig. 5.A 14-year-old boy with two fistulas; one showed discordant treatment response between transperineal ultrasonography (TPUS) and magnetic resonance imaging (MRI), and the other was not detected with TPUS.A. Sagittal TPUS shows a perianal fistula at the 12-o’clock location (arrow). B. Followup ultrasonography image after steroid and methotrexate treatment demonstrates no interval change in the fistula (arrow). C, E. On baseline MRI (sagittal and axial fat-suppressed T2-weighted images), the perianal fistula at 12 o’clock (arrow) corresponds with the TPUS finding. A second perianal fistula (arrowhead) is present at 6 o’clock, undetected using TPUS. Although both lesions are superficial, the 12-o’clock fistula is thicker than the 6-o’clock fistula. D, F. On follow-up sagittal and axial fatsuppressed T2-weighted images, the two perianal fistulas completely disappeared.
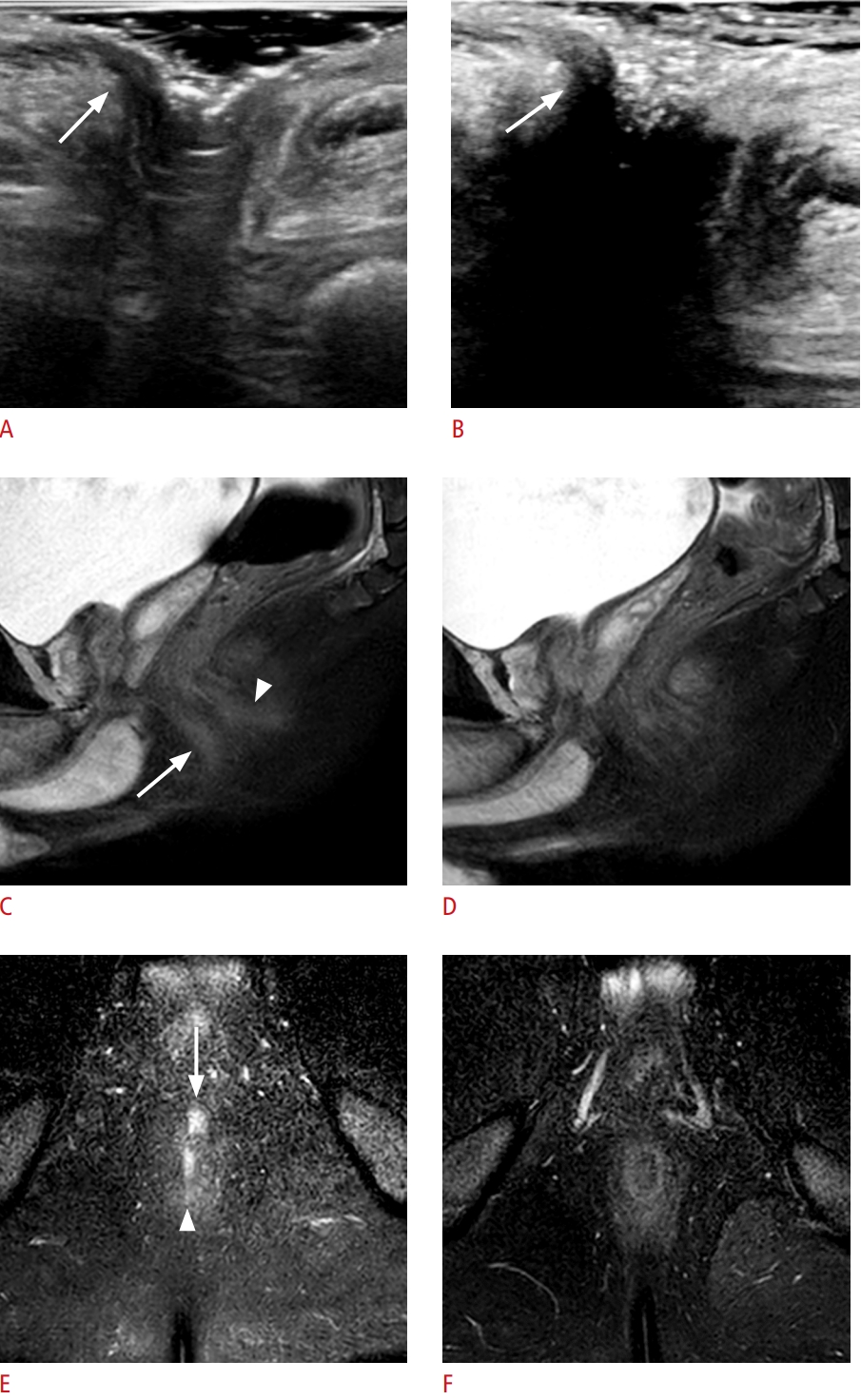 Fig. 6.Table 1.Demographics and clinical features of the study subjects Table 2.Numbers of perianal fistulas detected on MRI
Table 3.ROC curve analysis results for TPUS in evaluating the treatment response (positive vs. negative)
Table 4.Comparison of treatment response evaluations between pelvic MRI and TPUS Table 5.Comparison of baseline MRI findings between detected and undetected lesions on baseline TPUS
Values are presented as number (%) or mean±SD. MRI, magnetic resonance imaging; TPUS, transperineal ultrasonography; AGA, American Gastroenterological Association; SD, standard deviation. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||



 Print
Print facebook
facebook twitter
twitter Linkedin
Linkedin google+
google+

 Download Citation
Download Citation PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC




