AbstractPurposeThe relationship between contrast-enhanced ultrasound (CEUS) hemodynamics and the molecular biomarkers of adult-type diffuse gliomas, particularly isocitrate dehydrogenase (IDH), remains unclear. This study was conducted to provide a comprehensive description of the vascularization of adult-type diffuse gliomas using quantitative indicators. Additionally, it was designed to identify any variables with the potential to intraoperatively predict IDH mutation status.
MethodsThis prospective study enrolled patients with adult-type diffuse gliomas between November 2021 and September 2022. Intraoperative CEUS was performed, and CEUS videos were recorded for 90-second periods. Hemodynamic parameters, including the peak enhancement (PE) difference, were calculated based on the time-intensity curve of the region of interest. A differential analysis was performed on the CEUS parameters with respect to molecular biomarkers and grades. Receiver operating characteristic curves for various parameters were analyzed to evaluate the ability of those parameters to predict IDH mutation status.
ResultsSixty patients with adult-type diffuse gliomas were evaluated. All hemodynamic parameters, apart from rising time, demonstrated significant differences between IDH-mutant and IDH-wildtype adult-type diffuse gliomas. The PE difference emerged as the optimal indicator for differentiating between IDH-wildtype and IDH-mutant gliomas, with an area under the curve of 0.958 (95% confidence interval, 0.406 to 0.785). Additionally, the hemodynamic parameters revealed significant differences across both grades and types of adult-type diffuse gliomas.
ConclusionHemodynamic parameters can be used intraoperatively to effectively distinguish between IDH-wildtype and IDH-mutant adult-type diffuse gliomas. Additionally, quantitative CEUS equips neurosurgeons with dynamic perfusion information for various types and grades of adult-type diffuse gliomas.
Gliomas, which are the most common primary malignant tumors of the central nervous system [1], are categorized based on their cell of origin [2]. The 2021 World Health Organization Classification of Tumors of the Central Nervous System (CNS5) has brought about substantial changes in the classification of gliomas through the integration of molecular biomarkers [3,4]. Adult-type diffuse gliomas are divided into three categories: isocitrate dehydrogenase (IDH)ŌĆōmutant astrocytoma, IDH-mutant and 1p/19q-codeleted oligodendroglioma, and IDH-wildtype glioblastoma (GBM) [1]. Generally, IDH-mutant gliomas have a more favorable prognosis than IDH-wildtype tumors, leading to significant variations in surgical planning for adult-type diffuse gliomas with different IDH mutation statuses [5-10]. Although a combination of histology and genetic testing is the gold standard for the molecular detection and grading of diffuse gliomas, it requires intraoperative biopsy and postoperative pathological examination.
Radiomics, which employs magnetic resonance imaging (MRI), is a leading field of study for the preoperative prediction of IDH mutation status in gliomas. This approach enables the noninvasive assessment of intracranial lesions through the use of multiple imaging sequences [11,12]. More sophisticated techniques, such as high-resolution MRI diffusion imaging [13-17], are increasingly utilized to acquire functional imaging sequences. However, conventional methods such as contrast-enhanced MRI, despite their clinical utility in predicting IDH mutation status [8], provide only static perfusion information without temporal variation.
Contrast-enhanced ultrasonography (CEUS) can provide neurosurgeons with real-time information during surgical procedures by characterizing and highlighting brain tumors [18-20]. Compared to Doppler imaging, CEUS offers more comprehensive insights into dynamic perfusion and overall vascularization, enabling the real-time identification of blood-rich glioma lesions during neurosurgery. While some studies have described the morphological characteristics of different glioma grades using CEUS [19,21,22], the relationship between quantitative hemodynamic parameters and grades is not yet well understood. Furthermore, grading alone is insufficient for accurate classification, as molecular features also play a crucial role according to CNS5 guidelines. The IDH mutation status is highly correlated with glioma prognosis and can influence the extent of surgical resection [23-25]. At present, the hemodynamic characteristics associated with molecular biomarkers, particularly IDH mutation status, in adult-type diffuse gliomas remain unidentified.
It is crucial to distinguish the hemodynamics of neovascularization among the three subtypes of adult-type gliomas, particularly in GBM characterized by IDH-wildtype status. CEUS offers a distinct advantage due to its capacity to provide real-time imaging and dynamic perfusion assessment. Through the quantitative analysis of microcirculatory perfusion using time-intensity curves (TICs), CEUS offers valuable parameters such as peak enhancement (PE), regional blood flow (RBF), and the area under the TIC curve (S) [22,26].
This study was conducted to explore the potential of intraoperative CEUS in predicting the IDH mutation status and characterizing the vascularization of adult-type diffuse gliomas. Furthermore, the study was intended to explore the variations in hemodynamic parameters across different tumor types and grades.
This study protocol was approved by the Huashan Hospital Ethics Committee of Fudan University in Shanghai, China (approval No. 2021-838). The experiments were undertaken with the understanding and written consent of each participant, and the study conformed with the principles of the World Medical Association Declaration of Helsinki, published on the website of the Journal of the American Medical Association.
The protocol for this prospective study received approval from the institutional ethics review board of the authorsŌĆÖ affiliated institution. Before surgery, all patients provided informed consent, acknowledging the potential for CEUS examination during the procedure. Between November 2021 and September 2022, this study enrolled patients in the neurosurgical ward with a solitary lesion diagnosed as glioma based on preoperative MRI. The study included patients eligible for brain tumor resection, with no history of allergy to ultrasound contrast agents or neurological diseases like cerebrovascular disease, which could confound the study results. All tumors were singular and underwent intraoperative CEUS examination prior to tumor resection. In total, 66 patients were recruited, but four were pathologically confirmed as having non-glioma lesions (two pituitary tumors, one meningioma, and one metastatic breast cancer tumor). Additionally, two patients were excluded due to poor image quality. Ultimately, this study included 60 patients with a postoperative pathological diagnosis of adult-type diffuse glioma (Supplementary Fig. 1).
After bone flap removal, intraoperative ultrasound was performed epidurally. A microconvex probe (C2-7-VN, LOGIQ E9, GE Medical Systems, Milwaukee, WI, USA) was wrapped in a sterile sleeve and utilized to identify and locate the intracranial lesion prior to tumor resection [20,27]. Subsequently, the ultrasound contrast agent SonoVue (Bracco SpA, Milan, Italy) was injected intravenously (2.4 mL [5 mg/mL]) and flushed with 5 mL of normal saline. This allowed for the visualization of the lesion using a low mechanical index. The dynamic CEUS video was captured in the Digital Imaging and Communications in Medicine (DICOM) format with a static scan slice for approximately 90 seconds following the injection of the contrast agent. The CEUS parameters were set as follows: mechanical index=0.08, gain=60-65, and dynamic range=65-70. Time-gain compensation was centered for all measurements. The recorded CEUS videos were then transferred to a computer for the purpose of quantitative hemodynamic analysis (Fig. 1).
A computer expert programmed the script code necessary for quantitative analysis prior to surgery. This code operates within the MATLAB software (version 2021a, MathWorks Inc., Natick, MA, USA). After the CEUS videos (in DICOM format) were imported into the program, two data analysts, who were unaware of the pathological findings, manually outlined the region of interests (ROIs) in the CEUS images. Each CEUS video had three ROIs marked: the tumor parenchyma, the normal brain tissue at the same depth, and the area encompassing the entire lesion at peak enhancement. The TIC curves, representing the average intensity of the ROI over time, were plotted, and the hemodynamic parameters were automatically calculated. These parameters included PE, S, RBF, rising time (RT), rising slope (RS), and enhancement dispersion (ED). To minimize individual differences arising from varying lesion depths and contrast medium injection rates, the intensity of the tumor parenchyma was subtracted (yielding a difference) from the intensity of the brain tissue at the same depth for all parameters related to enhancement intensity. Specifically, the PE, S, and RBF of the tumor parenchyma and brain tissue at the same depth were subtracted, resulting in parameters identified as PE difference, S difference, and RBF difference. The parameters obtained by the two data analysts underwent a consistency assessment. The method for calculating the hemodynamic parameters is illustrated in Fig. 1.
The data were analyzed using SPSS (version 22.0, IBM Corp., Armonk, NY, USA), and a violin plot was generated using GraphPad Prism (version 8, GraphPad Software, San Diego, CA, USA). P-values of less than 0.05 were considered to indicate statistical significance. Adult-type diffuse gliomas were categorized based on their IDH mutation status, type, and grade. The Shapiro-Wilk normality test was used to check all hemodynamic parameters regarding normality of distribution. For parameters that did not follow a normal distribution, the Mann-Whitney U test was used to compare hemodynamic parameters between two groups, while the Kruskal-Wallis test with a post hoc Bonferroni correction was used for comparisons among three groups. For parameters that followed a normal distribution, the Student t-test was used for comparisons between two groups, and one-way analysis of variance with a post hoc Bonferroni correction was used for comparisons among three groups. The intraclass correlation coefficient was used to measure the consistency of the hemodynamic parameters obtained by the two data analysts.
Receiver operating characteristic (ROC) analysis was employed to determine the optimal cutoff values for distinguishing between IDH-mutant and IDH-wildtype gliomas. The thresholds yielding the highest Youden index were designated as these cutoff values. Additionally, the area under the curve (AUC), along with the sensitivity and specificity, was calculated using these optimal cutoffs.
This study included 60 patients (28 men and 32 women) with adult-type diffuse gliomas, with an average age of 49.7┬▒14.4 years. Participants were categorized based on their postoperative pathological results (CNS5). Based on grade, 18 grade 2 gliomas were classified as low-grade glioma (LGG; n=18), whereas 13 grade 3 gliomas and 29 grade 4 gliomas were grouped as high-grade glioma (HGG; n=42). The adult-type diffuse gliomas were further categorized by type: astrocytoma, IDH-mutant (n=20); oligodendroglioma, IDH-mutant and 1p/19q-coldeleted (n=7); or GBM, IDH-wildtype (n=33). Additionally, the gliomas were grouped based on IDH mutation status, either as IDH-mutant (n=27) or IDH-wildtype (n=33). The demographic characteristics of the patients with adult-type diffuse gliomas are summarized in Table 1.
A consistency analysis was conducted to assess the reliability of the continuous variables collected by the two data analysts. The intraclass correlation coefficient was used to test the data, revealing that all hemodynamic parameters had intraclass correlation coefficients greater than 0.75 between the analysts (Table 2) (all P<0.001). This indicates that the hemodynamic parameters were consistent between the two analysts, affirming the validity of the results.
A differential analysis of the hemodynamic parameters between IDH-mutant and IDH-wildtype gliomas was conducted. Parameters including PE difference, S difference, RBF difference, RS, and ED, which were derived from the TICs, were found to distinguish IDH-mutant from IDH-wildtype adult-type diffuse gliomas (all P<0.001). These parameters were significantly higher in IDH-wildtype gliomas than in IDH-mutant gliomas. The results of the statistical tests are presented in Fig. 2 and Supplementary Table 1. No significant difference was observed in the RT between IDH-mutant and IDH-wildtype adult-type diffuse gliomas (P=0.109). As a result, this parameter was not included in the subsequent analysis of diagnostic efficiency.
To evaluate the diagnostic efficacy of hemodynamic parameters in distinguishing between IDH-mutant and IDH-wildtype tumors, an ROC analysis was performed. The results of this analysis, along with 95% confidence intervals (CIs), are presented in Table 3. The parameters of PE difference, S difference, RBF difference, RS, and ED were effective in distinguishing IDH-mutant from IDH-wildtype adult-type diffuse gliomas (all P<0.001). The highest AUC was noted for PE difference (AUC for PE difference: 0.908 [95% CI, 0.406 to 0.785]; for S difference: 0.880 [95% CI, 0.770 to 0.950]; for RBF difference: 0.889 [95% CI, 0.781 to 0.955]; for RS: 0.846 [95% CI, 0.730 to 0.926]; and for ED: 0.827 [95% CI, 0.708 to 0.913]). The cutoff values for PE difference, S difference, RBF difference, RS, and ED were 1.71, 538.00, 1.77, 0.36, and 230, respectively. The respective sensitivity and specificity values for PE difference were 92.6% and 81.8%; for S difference, they were 88.9% and 72.7%; for RBF difference, they were 88.9% and 81.8%; for RS, they were 92.6% and 78.8%; and for ED, they were 81.5% and 87.9% (Supplementary Fig. 2). Examples of PE difference, as well as CEUS images and immunohistochemistry, of IDH-wildtype and IDH-mutant adult-type diffuse gliomas are displayed in Figs. 3 and 4.
A differential analysis was conducted on the hemodynamic parameters in astrocytoma (IDH-mutant), oligodendroglioma (IDH-mutant and 1p/19q-coldeleted), and GBM (IDH-wildtype). The S difference, RBF difference, and RS were significantly higher in GBM (IDH-wildtype) compared to oligodendroglioma (IDH-mutant and 1p/19q-coldeleted) (S difference: P=0.001; others: P<0.001). In contrast, RT was significantly higher in oligodendroglioma (IDH-mutant and 1p/19q-coldeleted) (P=0.035). Furthermore, the PE difference, S difference, RBF difference, RS, and ED were significantly higher in GBM (IDH-wildtype) compared to astrocytoma (IDH-mutant) (all P<0.001). ED was the only parameter that demonstrated a significant difference between astrocytoma (IDH-mutant) and oligodendroglioma (IDH-mutant and 1p/19q-coldeleted). The means and standard deviations of these parameters in adult-type diffuse gliomas are detailed in Table 4. The results of the differential analysis in adult-type diffuse gliomas are illustrated in Fig. 2 and Supplementary Table 1.
Not all parameters effectively differentiated between LGGs and HGGs. However, significant differences were observed in the PE difference, S difference, RT, and RS between LGG and HGG. The PE difference, S difference, and RS were significantly higher in HGG compared to LGG, while RT was significantly higher in LGG (all P<0.05). Furthermore, these parametersŌĆöPE difference, S difference, RT, and RSŌĆöalso varied among grade 2, 3, and 4 adult-type diffuse gliomas (all P<0.05). ED, however, showed no significant difference across grades. The results of the difference analysis across various grades are illustrated in Fig. 2.
CEUS is a dependable instrument for intraoperative visualization of the vascularity of focal lesions. Meves et al. [26] suggested that quantitative methods could be applied to interpret the hemodynamics of CEUS. To date, seven studies have employed CEUS for intraoperative navigation and diagnosis of adult-type diffuse gliomas [19,21,22,28-31]. Most of these studies primarily focused on morphology and grading. The present study explored the feasibility of using CEUS hemodynamic parameters to detect IDH mutations. Furthermore, variations in these parameters were scrutinized in different types and grades of adult-type diffuse gliomas.
As opposed to the existing quantitative CEUS analysis software [22], this study utilized the MATLAB software, which allows independent programming. This enables data analysts to write scripts that define and incorporate quantitative parameters based on specific research needs. In clinical practice, the authors have found that the intensity of brain tissue on CEUS may relate to the patientŌĆÖs age and underlying conditions. To minimize the bias of these individual differences in hierarchical diagnosis, the intensity-related parameters were subtracted between the tumor parenchyma and the brain tissue at the same depth. Furthermore, necrosis in gliomas has long been recognized as an important feature of malignancy [32]. To quantitatively examine its correlation with molecular pathological characteristics, particularly IDH, the parameter of ED was incorporated (Fig. 1).
In 2014, Prada et al. [19] demonstrated an association between a higher degree of CEUS enhancement in tumors and an increased risk of malignancy, such as GBM, based on the cell origin of adult-type diffuse gliomas. According to CNS5 guidelines, molecular biomarkers such as IDH serve as an accurate method for prognosis prediction and treatment planning. The vasculature is a highly dynamic, tissue-specific organ [33]. IDH-wildtype gliomas are distinguished by a higher degree of microvascular proliferation than IDH-mutant gliomas [34-37]. The effectiveness of antiangiogenic therapy (bevacizumab) in IDH-wildtype glioma suggests that these gliomas may exhibit greater hemodynamic heterogeneity with brain tissue compared to IDH-mutant gliomas. This study employed an objective method to measure regional microvascular hemodynamics. The results indicated that parameters such as PE difference, S difference, RBF difference, and RS could serve as effective indicators for differentiating IDH-mutant tumors from IDH-wildtype tumors (Figs. 3, 4). Of these, PE difference displayed the highest AUC in ROC tests (Table 4). PE represents the peak concentration of contrast agent in the vascular lumen, which is associated with the density of microvessels [37]. PE difference is the most intuitive parameter reflecting the heterogeneity of blood pool volume between the tumor and normal brain tissue. Therefore, IDH-wildtype adult-type diffuse gliomas exhibit a greater difference in proliferation between the tumor parenchyma and the normal brain tissue. A higher PE difference value can distinguish IDH-wildtype from IDH-mutant adult-type diffuse gliomas. However, due to varying enhancement times in patients, S difference, RBF difference, and RS were less effective than PE difference.
The differential analysis results of hemodynamic parameters in molecular pathologic classification and grading partially corroborate the aforementioned assumption that glioma malignancy correlates with hemodynamic heterogeneity. This research demonstrated that the majority of CEUS hemodynamic parameters varied significantly between astrocytoma (IDH-mutant) and GBM (IDH-wildtype), as well as between oligodendroglioma (IDH-mutant and 1p/19q-coldeleted) and GBM (IDH-wildtype). Similar findings were obtained between LGG and HGG. This is attributable to the fact that IDH serves as a crucial molecular biomarker in differentiating various types of adult-type diffuse gliomas. Furthermore, IDH-wildtype gliomas typically display malignancy and poor prognosis, predominantly characterizing high-grade gliomas [4]. However, only ED exhibited a significant difference between oligodendroglioma (IDH-mutant and 1p/19q-coldeleted) and astrocytoma (IDH-mutant) (Table 3).
This study had several limitations. First, the intraoperative CEUS examination and postoperative ROI selection were dependent on the operator. To mitigate this, ultrasound was conducted following flap removal to improve image quality, and multiple measurements were taken and averaged to reduce potential observer error. As another limitation, a hemodynamic quantitative analysis must be carried out following the CEUS examination, and real-time classification has not yet been accomplished. Despite this, the method described herein is sufficiently convenient to provide the surgeon with hemodynamic information prior to resection. Additionally, the number of cases included in the study was relatively small, particularly for oligodendroglioma (IDH-mutant and 1p/19q-coldeleted). As such, these results should be confirmed through prospective multicenter studies.
The present study demonstrated that CEUS provides neurosurgeons with information related to dynamic perfusion of the lesion. This information can be used intraoperatively to effectively differentiate between IDH-wildtype and IDH-mutant gliomas. CEUS may aid in the noninvasive assessment for treatment planning and prognosis prediction of adult-type diffuse gliomas. Additionally, differences were observed in hemodynamic parameters among patients with various types and grades of adult-type diffuse gliomas.
NotesAuthor Contributions Conceptualization: Shi Z, Wang Y, Yu J, Ding H. Data acquisition: Zhang X, Shi Z, Shen C, Qi Z, Zhang L, Yang B. Data analysis or interpretation: Zhang X, Xie Y. Drafting of the manuscript: Zhang X. Critical revision of the manuscript: Zhang X, Shi Z, Xie Y, Wang Y, Shen C, Qi Z, Zhang L, Yang B, Yu J, Ding H. Approval of the final version of the manuscript: all authors. Conflict of InterestThis work was financially supported by the National Natural Science Foundation of China (No. 82272017). The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper. Supplementary MaterialSupplementary Table 1.P-values for tests in IDH mutation status, types and grades of adult-type diffuse gliomas (https://doi.org/10.14366/usg.23031).
Supplementary Fig. 1.Flow chart of the implementation of this study (https://doi.org/10.14366/usg.23031).
Supplementary Fig. 2.ROC curve comparison of hemodynamic parameters for distinguishing between IDH-mutant and IDH-wildtype adult-type diffuse gliomas (https://doi.org/10.14366/usg.23031).
References1. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO Classification of tumors of the central nervous system: a summary. Neuro Oncol 2021;23:1231ŌĆō1251.
2. Weller M, Wick W, Aldape K, Brada M, Berger M, Pfister SM, et al. Glioma. Nat Rev Dis Primers 2015;1:15017.
3. Sledzinska P, Bebyn MG, Furtak J, Kowalewski J, Lewandowska MA. Prognostic and predictive biomarkers in gliomas. Int J Mol Sci 2021;22:10373.
4. Wen PY, Packer RJ. The 2021 WHO classification of tumors of the central nervous system: clinical implications. Neuro Oncol 2021;23:1215ŌĆō1217.
5. Molinaro AM, Wiencke JK, Warrier G, Koestler DC, Chunduru P, Lee JY, et al. Interactions of age and blood immune factors and noninvasive prediction of glioma survival. J Natl Cancer Inst 2022;114:446ŌĆō457.
6. Tesileanu CM, Dirven L, Wijnenga MMJ, Koekkoek JAF, Vincent A, Dubbink HJ, et al. Survival of diffuse astrocytic glioma, IDH1/2 wildtype, with molecular features of glioblastoma, WHO grade IV: a confirmation of the cIMPACT-NOW criteria. Neuro Oncol 2020;22:515ŌĆō523.
7. Hartmann C, Hentschel B, Wick W, Capper D, Felsberg J, Simon M, et al. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol 2010;120:707ŌĆō718.
8. Park YW, Vollmuth P, Foltyn-Dumitru M, Sahm F, Ahn SS, Chang JH, et al. The 2021 WHO classification for gliomas and implications on imaging diagnosis: part 1-key points of the fifth edition and summary of imaging findings on adult-type diffuse gliomas. J Magn Reson Imaging 2023;58:677ŌĆō689.
10. Eckel-Passow JE, Lachance DH, Molinaro AM, Walsh KM, Decker PA, Sicotte H, et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med 2015;372:2499ŌĆō2508.
11. Zhang J, Cao J, Tang F, Xie T, Feng Q, Huang M. Multi-level feature exploration and fusion network for prediction of IDH status in gliomas from MRI. IEEE J Biomed Health Inform 2023 May 29 [Epub]. https://doi.org/10.1109/JBHI.2023.3279433.
12. Yan J, Zhang B, Zhang S, Cheng J, Liu X, Wang W, et al. Quantitative MRI-based radiomics for noninvasively predicting molecular subtypes and survival in glioma patients. NPJ Precis Oncol 2021;5:72.
13. Zhang L, Yang LQ, Wen L, Lv SQ, Hu JH, Li QR, et al. Noninvasively evaluating the grading of glioma by multiparametric magnetic resonance imaging. Acad Radiol 2021;28:e137ŌĆōe146.
14. Wang P, Weng L, Xie S, He J, Ma X, Li B, et al. Primary application of mean apparent propagator-MRI diffusion model in the grading of diffuse glioma. Eur J Radiol 2021;138:109622.
15. Luan J, Wu M, Wang X, Qiao L, Guo G, Zhang C. The diagnostic value of quantitative analysis of ASL, DSC-MRI and DKI in the grading of cerebral gliomas: a meta-analysis. Radiat Oncol 2020;15:204.
16. Li S, Zheng Y, Sun W, Lasic S, Szczepankiewicz F, Wei Q, et al. Glioma grading, molecular feature classification, and microstructural characterization using MR diffusional variance decomposition (DIVIDE) imaging. Eur Radiol 2021;31:8197ŌĆō8207.
17. Zaccagna F, Riemer F, Priest AN, McLean MA, Allinson K, Grist JT, et al. Non-invasive assessment of glioma microstructure using VERDICT MRI: correlation with histology. Eur Radiol 2019;29:5559ŌĆō5566.
18. Unsgaard G, Gronningsaeter A, Ommedal S, Nagelhus Hernes TA. Brain operations guided by real-time two-dimensional ultrasound: new possibilities as a result of improved image quality. Neurosurgery 2002;51:402ŌĆō411.
19. Prada F, Perin A, Martegani A, Aiani L, Solbiati L, Lamperti M, et al. Intraoperative contrast-enhanced ultrasound for brain tumor surgery. Neurosurgery 2014;74:542ŌĆō552.
20. Prada F, Del Bene M, Mauri G, Lamperti M, Vailati D, Richetta C, et al. Dynamic assessment of venous anatomy and function in neurosurgery with real-time intraoperative multimodal ultrasound: technical note. Neurosurg Focus 2018;45:E6.
21. Prada F, Mattei L, Del Bene M, Aiani L, Saini M, Casali C, et al. Intraoperative cerebral glioma characterization with contrast enhanced ultrasound. Biomed Res Int 2014;2014:484261.
22. Cheng LG, He W, Zhang HX, Song Q, Ning B, Li HZ, et al. Intraoperative contrast enhanced ultrasound evaluates the grade of glioma. Biomed Res Int 2016;2016:2643862.
23. Beiko J, Suki D, Hess KR, Fox BD, Cheung V, Cabral M, et al. IDH1 mutant malignant astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neuro Oncol 2014;16:81ŌĆō91.
24. Patel T, Bander ED, Venn RA, Powell T, Cederquist GY, Schaefer PM, et al. The role of extent of resection in IDH1 wild-type or mutant low-grade gliomas. Neurosurgery 2018;82:808ŌĆō814.
25. Patel SH, Bansal AG, Young EB, Batchala PP, Patrie JT, Lopes MB, et al. Extent of surgical resection in lower-grade gliomas: differential impact based on molecular subtype. AJNR Am J Neuroradiol 2019;40:1149ŌĆō1155.
26. Meves SH, Wilkening W, Thies T, Eyding J, Holscher T, Finger M, et al. Comparison between echo contrast agent-specific imaging modes and perfusion-weighted magnetic resonance imaging for the assessment of brain perfusion. Stroke 2002;33:2433ŌĆō2437.
27. Rubin JM, Mirfakhraee M, Duda EE, Dohrmann GJ, Brown F. Intraoperative ultrasound examination of the brain. Radiology 1980;137:831ŌĆō832.
28. Arlt F, Chalopin C, Muns A, Meixensberger J, Lindner D. Intraoperative 3D contrast-enhanced ultrasound (CEUS): a prospective study of 50 patients with brain tumours. Acta Neurochir (Wien) 2016;158:685ŌĆō694.
29. He W, Jiang XQ, Wang S, Zhang MZ, Zhao JZ, Liu HZ, et al. Intraoperative contrast-enhanced ultrasound for brain tumors. Clin Imaging 2008;32:419ŌĆō424.
30. Prada F, Vitale V, Del Bene M, Boffano C, Sconfienza LM, Pinzi V, et al. Contrast-enhanced MR imaging versus contrast-enhanced US: a comparison in glioblastoma surgery by using intraoperative fusion imaging. Radiology 2017;285:242ŌĆō249.
31. Prada F, Del Bene M, Mattei L, Lodigiani L, DeBeni S, Kolev V, et al. Preoperative magnetic resonance and intraoperative ultrasound fusion imaging for real-time neuronavigation in brain tumor surgery. Ultraschall Med 2015;36:174ŌĆō186.
33. Jhaveri N, Chen TC, Hofman FM. Tumor vasculature and glioma stem cells: contributions to glioma progression. Cancer Lett 2016;380:545ŌĆō551.
35. Huse JT, Holland EC. Targeting brain cancer: advances in the molecular pathology of malignant glioma and medulloblastoma. Nat Rev Cancer 2010;10:319ŌĆō331.
Quantitative hemodynamic analysis and parameter definition.A, B. The schematic illustrates the process of establishing regions of interest (ROIs) in contrast-enhanced ultrasonography. (1) An ROI was selected within the parenchyma of the glioma lesion. (2) Another ROI was chosen in the brain tissue at an equivalent depth, avoiding large blood vessels. (1) A third ROI was determined that included the entire glioma lesion at PE. ROIs were delineated based on the lesions identified in the grayscale image (left), and the same ROI outline is displayed in the contrast-enhanced ultrasound image (right). C, D. The red and blue curves represent the average intensity over time for ROI (1) and ROI (2), respectively, forming the time-intensity curve (TIC) and allowing for calculations of hemodynamic parameters through the TIC. tp is defined as the time corresponding to the peak, t0 as the onset of enhancement, and t1 as the end of enhancement. ROI (3) includes the entire glioma lesion at its peak, and enhancement dispersion (ED) represents the difference between the maximum and minimum intensity within the ROI. PE, peak enhancement; RBF, regional blood flow; RS, rising slope; RT, rising time; S, area under curve.
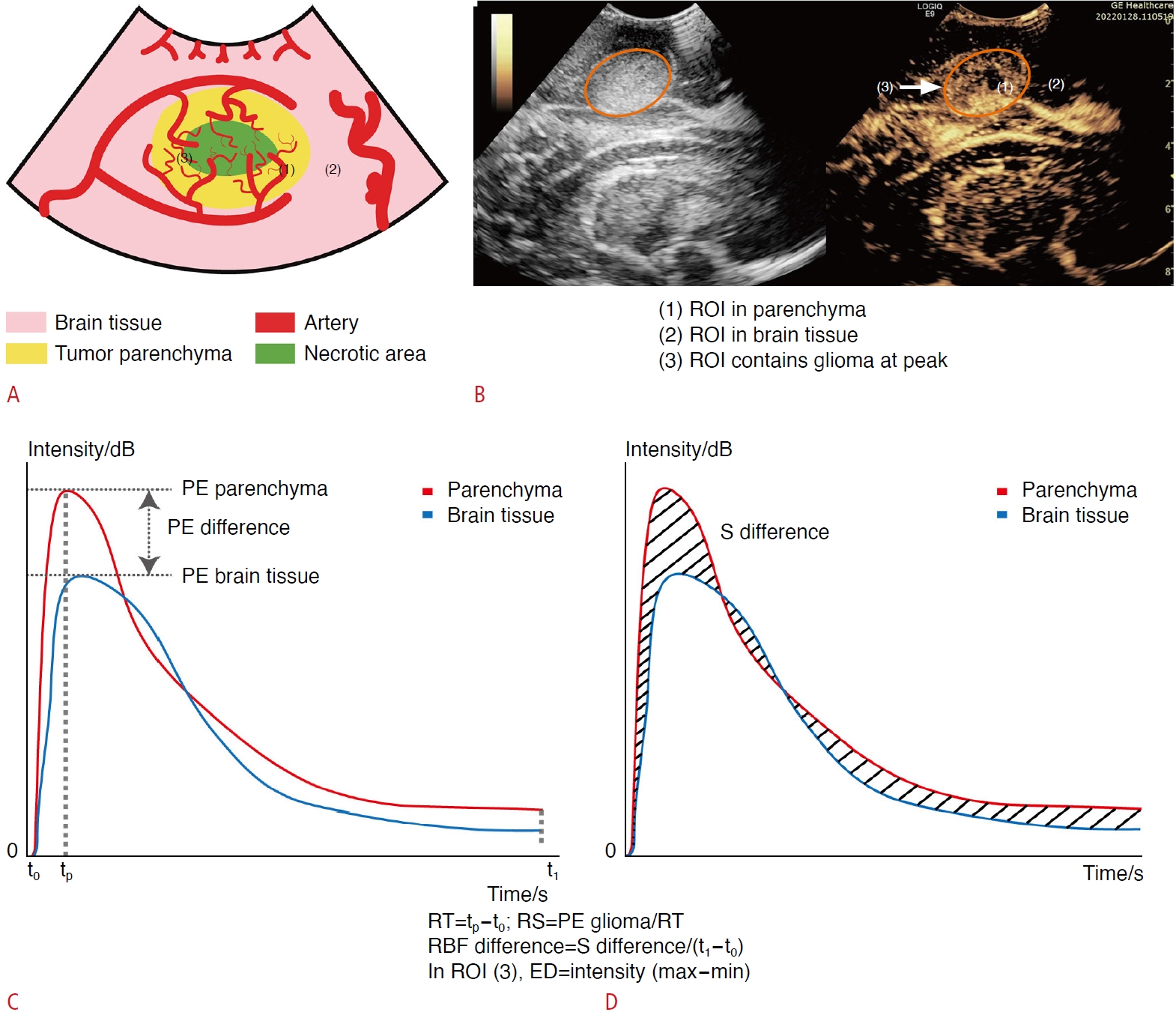 Fig.┬Ā1.Violin plots of hemodynamic parameters according to grade, type, and IDH mutation status.Differential analysis of quantitative parameters is shown: (A) PE difference, (B) S difference, (C) RT, (D) RBF difference, (E) RS, and (F) ED, in relation to IDH mutation status, glioma types, and grades in supratentorial adult-type diffuse gliomas using contrast-enhanced ultrasound. ED, enhancement dispersion; GBM, glioblastoma; HGG, high-grade glioma; IDH, isocitrate dehydrogenase; LGG, low-grade glioma; PE, peak enhancement; RBF, regional blood flow; RS, rising slope; RT, rising time; S, area under curve. *P<0.05 were considered to indicate statistical significance.
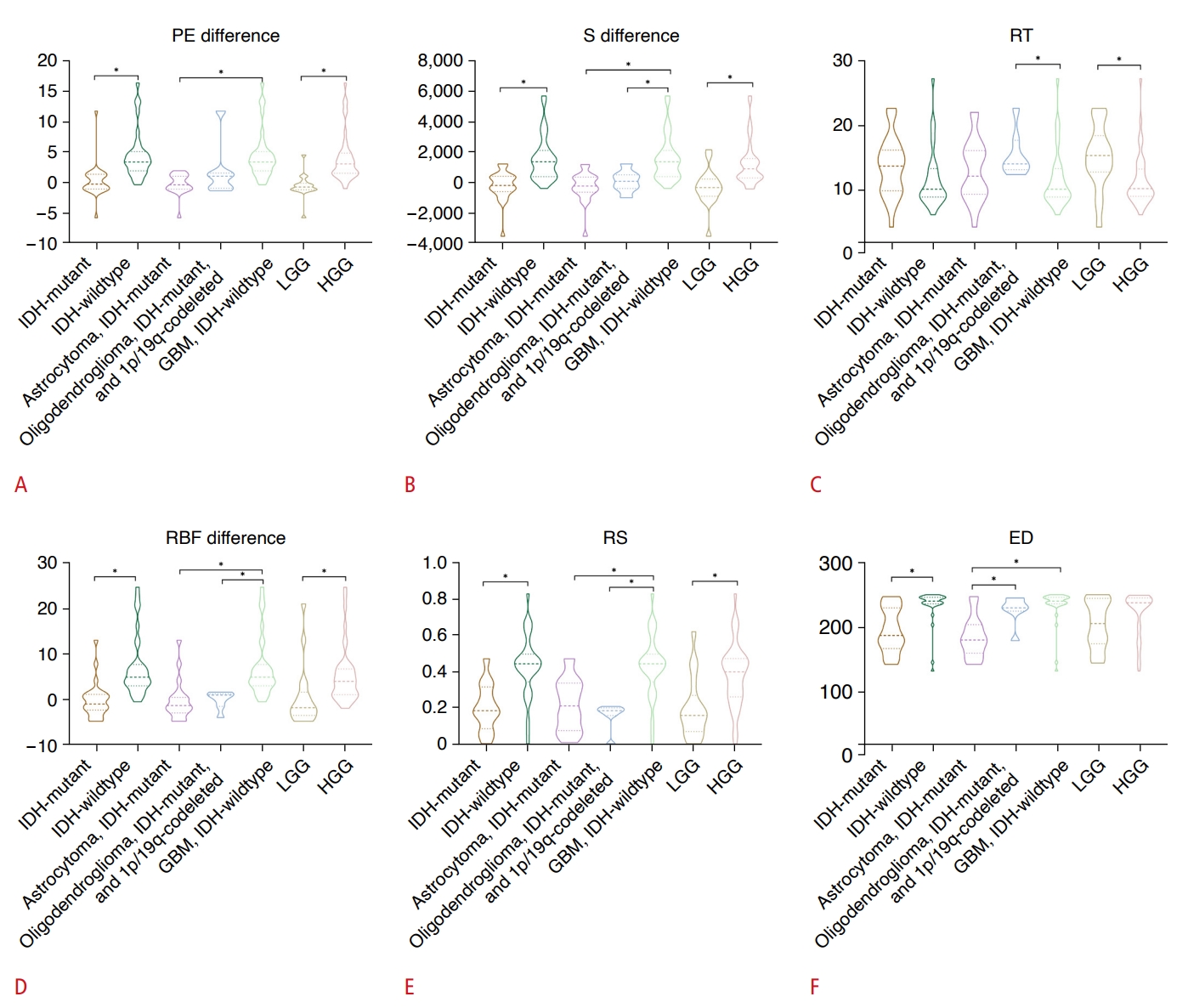 Fig.┬Ā2.Peak enhancement difference in isocitrate dehydrogenase (IDH)-wildtype adult-type diffuse gliomas.A 53-year-old woman was diagnosed with a high-grade glioma in the left lateral ventricle based on preoperative magnetic resonance imaging (MRI). A. Postoperative immunohistochemical analysis confirmed the diagnosis as a glioblastoma, IDH-wildtype (IDH1, ├Ś200). B, C. Contrast-enhanced MRI and grayscale ultrasound reveals that the lesion was in the left lateral ventricle (The yellow circle represents the approximate extent of the lesion). D. Basline enhancement at the beginning of recording contrast-enhanced ultrasound video (time=0 s). E, F. The peak enhancement (PE) of the tumor and of the brain tissue at the same depth, respectively. The enhancement of the tumor parenchyma at PE was significantly stronger than that of the brain tissue. G. The regions of interest (ROIs) were delineated in the tumor parenchyma (red circle) and brain tissue (blue circle) at the same depth to generate the time-intensity curve. H. The color of the curve corresponded to the color of the ROI in G. The PE on the time-intensity curve was distinct between the glioma parenchyma and the brain tissue, with a PE difference of 13.99.
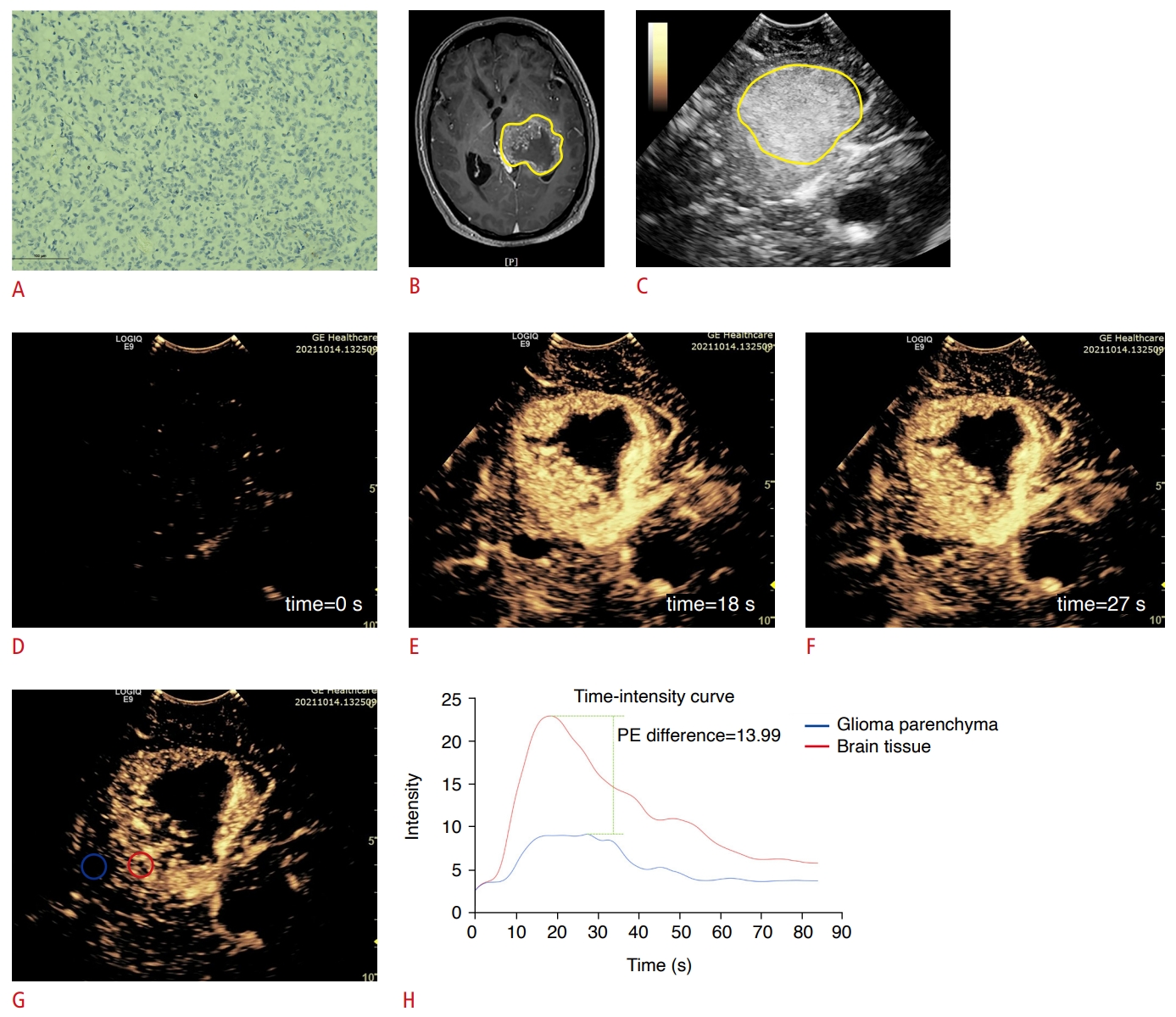 Fig.┬Ā3.Peak enhancement difference in isocitrate dehydrogenase (IDH)-mutant adult-type diffuse gliomas.A 55-year-old woman was diagnosed with a high-grade glioma in the right frontal lobe based on preoperative magnetic resonance imaging (MRI). A. Postoperative immunohistochemical analysis confirmed the diagnosis as an astrocytoma, IDH-mutant (IDH1, ├Ś200). B, C. Contrast-enhanced MRI and grayscale ultrasound reveals that the lesion was in the right frontal lobe (The yellow circle represents the approximate extent of the lesion). D. Basline enhancement at the beginning of recording contrast-enhanced ultrasound video (time=0 s). E, F. The peak enhancement (PE) of the tumor and of the brain tissue at the same depth, respectively. The enhancement of the tumor parenchyma at its peak was close to that of the brain tissue. G. The regions of interest (ROIs) were delineated in the tumor parenchyma (red circle) and the brain tissue (blue circle) at the same depth to generate a time-intensity curve. H. The color of the curve corresponded to the color of the ROI in G. The PE on the time-intensity curve was slightly higher in the brain tissue than in the glioma parenchyma, with a PE difference of -0.89.
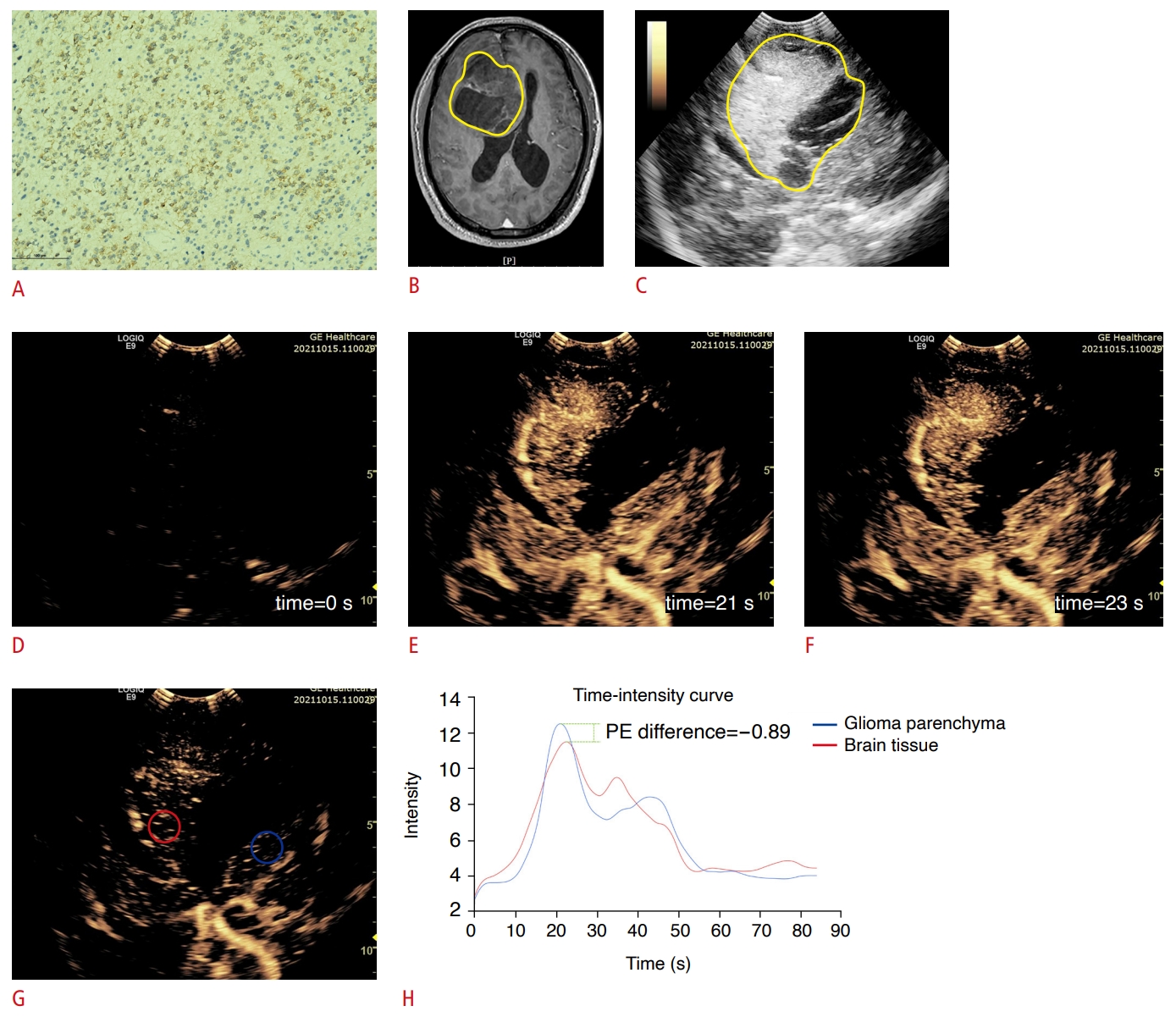 Fig.┬Ā4.Table┬Ā1.Demographic characteristics of patients with adult-type diffuse gliomas Table┬Ā2.Consistency test of hemodynamic parameters acquired by two data analysts
Table┬Ā3.ROC analysis of hemodynamic parameters for distinguishing between IDH-mutant and IDH-wildtype adult-type diffuse gliomas P-values <0.05 were considered to indicate statistical significance. ROC, receiver operating characteristic; IDH, isocitrate dehydrogenase; AUC, area under the ROC curve; CI, confidence interval; PE, peak enhancement; S, area under curve; RBF, regional blood flow; RS, rising slope; ED, enhancement dispersion. Table┬Ā4.Hemodynamic parameters (mean┬▒SD) by grade and type of adult-type diffuse gliomas |



 Print
Print facebook
facebook twitter
twitter Linkedin
Linkedin google+
google+
 Download Citation
Download Citation PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI




