AbstractPurposeThe utility of contrast-enhanced ultrasound (CEUS)–guided liver biopsy in patients with treated hepatocellular carcinoma (HCC) was evaluated.
MethodsThis study prospectively enrolled 36 patients (mean age±standard deviation [SD], 65.3±9.2 years; 31 men) who underwent CEUS-guided biopsy for treated HCC between September 2020 and April 2022, constituting the CEUS group. An additional 60 patients (mean age±SD, 60.7±12.3 years; 50 men) who underwent B-mode image-guided biopsy for treated HCC between January 2017 and December 2018 were retrospectively enrolled in the B-mode, or control, group. Biopsy success rates were compared between CEUS and B-mode groups using the chi-square test.
ResultsThe biopsy success rate in the CEUS group was 88.9% (32 of 36 patients), which was significantly higher than the 70.0% (42 of 60 patients) observed in the B-mode group (P=0.044). No significant difference was found between CEUS and B-mode groups in the size of the lesions targeted for biopsy (mean±SD, 3.8±2.3 cm vs. 3.7±3.3 cm, respectively; P=0.332). For both the whole tumor and the viable tumor, conspicuity scores were significantly higher on CEUS than on grayscale ultrasound in the CEUS group (whole tumor: 2.7±0.5 vs. 1.8±0.9, P<0.001; viable tumor: 2.6±0.7 vs. 1.4±0.8, P<0.001). Relative to non-diagnostic results, diagnostic results more frequently exhibited a late mild washout pattern (87.5% [28 of 32] vs. 25.0% [1 of 4], P=0.004). No significant difference in the arterial enhancement pattern was noted between these subgroups (P=0.415).
Unlike other malignancies that require histopathological examination for definitive diagnosis, hepatocellular carcinoma (HCC) can be diagnosed in high-risk patients through noninvasive imaging studies. Most current clinical practice guidelines for HCC include noninvasive diagnostic algorithms tailored for specific risk groups. These algorithms are based on characteristic imaging features, such as arterial phase hyperenhancement and washout in the portal venous and/or delayed phases on multiphasic computed tomography (CT) or magnetic resonance imaging (MRI) [1-4]. The feasibility of noninvasive HCC diagnosis via imaging largely stems from the sufficient pretest probability of HCC in patients with cirrhosis, coupled with the high specificity and positive predictive value of the imaging criteria used in the targeted screening cohort [5]. Additionally, liver biopsy carries risks of bleeding, tumor seeding, and the potential for non-diagnostic results [6].
In the era of precision medicine, clinical demand exists for tissue acquisition as part of the treatment of advanced HCC through clinical trials, genomic or molecular analysis, and biomarker development [7]. Following the success of the IMbrave150 trial, which demonstrated that atezolizumab combined with bevacizumab facilitated significantly better overall survival than sorafenib [8], many clinical trials for systemic chemotherapy in HCC now require tissue confirmation for eligibility [9]. Generally, the diagnostic yield of percutaneous ultrasound (US)-guided targeted biopsy of focal liver lesions is high, particularly for tumors larger than 1 cm, with success rates ranging from 85.1% to 95.2% [10-12]. However, given that most patients with HCC have already undergone treatments of various modalities—including surgical resection, locoregional therapies such as ablation, transarterial chemoembolization (TACE), and radiation therapy—before transitioning to systemic therapy or enrolling in clinical trials, differentiating viable tumor tissue from necrotic tissue using B-mode imaging for US-guided targeted biopsy can be challenging.
To address the limitations of B-mode US-guided liver biopsy, several previous studies have demonstrated that contrast-enhanced ultrasound (CEUS) facilitates the accurate identification of the target lesion, thereby improving the diagnostic yield of liver biopsy, even for small lesions not visible on B-mode US [13-15]. Additionally, CEUS has been demonstrated to be useful in evaluating treatment response and detecting residual HCC following locoregional therapies [16]. This study was designed to prospectively assess the clinical utility of CEUS-guided liver biopsy in patients with treated HCC.
The relevant institutional review board (No. 2002-052-1100) approved this prospective study. Informed consent was obtained from all prospectively enrolled participants before they underwent CEUS and biopsy procedures. Additionally, to compare the efficacy of CEUS-guided biopsy in treated HCC, a historical control group of patients was retrospectively selected. These individuals had previously undergone liver biopsy for HCC treatment under B-mode US guidance, without the use of contrast. For this control group, the requirement to obtain informed consent was waived due to the retrospective nature of the study.
Between September 2020 and April 2022, 37 participants were consecutively enrolled if they met the following inclusion criteria: (1) over 20 years old; (2) diagnosed with HCC based on noninvasive criteria or histopathologic analysis; (3) underwent surgery, chemoembolization, radiofrequency ablation (RFA), radiation therapy, radioembolization, or systemic therapy for HCC; and (4) referred to the radiology department for liver biopsy of treated HCC (Fig. 1). Of the 37 participants, one was excluded because the target lesion was not visible on B-mode US or CEUS due to interference from the lung shadow, resulting in an inadequate acoustic window; consequently, no biopsy was performed.
According to a search of the radiology database of the authors’ institution, 1,946 patients underwent liver biopsy between January 2017 and December 2018 (Fig. 1). Of these patients, 203 underwent the procedure due to suspected HCC. A subset of 66 consecutive patients met the inclusion criteria for the CEUS group. For patients who underwent two liver biopsy procedures during the study period (n=6), only the initial result was considered for inclusion in the study. After removing six patients who underwent CEUS-guided liver biopsy, the remaining 60 patients comprised the B-mode US group. All biopsies in this group were performed by one of five board-certified radiologists, each with a minimum of 4 years of experience in abdominal ultrasonography and involvement in over 200 percutaneous liver biopsy cases.
Real-time CEUS was conducted by one of two radiologists, each with substantial experience in CEUS (12 and 4 years, with a minimum of 300 percutaneous liver biopsy cases). The procedure utilized a contrast-specific US system (Aplio i800, Canon Medical Systems, Tochigi, Japan) equipped with a wideband convex i8CX1 multi-frequency probe. Prior to the examination, patients were required to fast for at least 6 hours. The CEUS images were captured in contrast-specific US mode using the following settings: contrast harmonic frequency, 3.0 MHz; mechanical index, 0.09; dynamic range, 60 dB; gain, 76 dB; and frame rate, 12 frames per second. Intravenous bolus injection of 2.4 mL of SonoVue (Bracco Imaging S.p.A., Milan, Italy) through the antecubital vein was followed by a 10-mL flush of normal saline. Continuous CEUS imaging of the target lesion was then performed during calm, normal respiration for the initial 60 seconds post-injection. This was followed by intermittent scanning, with each scan lasting 5 seconds, at 30-second intervals for a total duration of 5 minutes.
All biopsies were conducted by the same radiologist who performed the CEUS procedures. The biopsies were carried out using an 18-gauge, automated, side-cutting needle (Acecut, TSK Laboratory, Tochigi, Japan) and the freehand technique. Patients received local anesthesia with 2% lidocaine hydrochloride. A minimum of two biopsy specimens, each 20 mm in length, were obtained. Additional samples were taken if the visual inspection of the specimen raised doubts about the technical success of the procedure.
All biopsy specimens were fixed in formalin and embedded in paraffin blocks. Hematoxylin and eosin staining was then performed, with additional immunohistochemical staining, such as cytokeratin 19, used in challenging cases. If the histopathologic report indicated that a specimen did not permit a specific diagnosis due to insufficient content (e.g., nonspecific specimen or necrotic cells) or mistargeting (e.g., non-neoplastic liver parenchyma), the result was classified as non-diagnostic [17]. The biopsy success rate was calculated using the following formula: (total number of biopsies-number of non-diagnostic results)/total number of biopsies. Treatment methods employed following biopsy were retrieved from electronic medical records.
Real-time imaging fusion of CT or MR images with B-mode US was selectively performed using the fusion algorithm embedded in the US system (Smart Fusion, Canon Medical Systems) in cases where CT or MRI-determined target lesions were not clearly visualized on US. Initially, the operator selected the CT or MR images for fusion that best depicted the target lesion and vascular anatomy, then transferred these to the US system via Digital Imaging Communications in Medicine data. Subsequently, when the operator positioned the US probe in the epigastric area in the sagittal plane, the fusion algorithm provided the position and orientation of the probe relative to the patient’s body within the transmitter’s spatial volume [18]. Ultimately, the operator aligned the vascular structures in the US image with the corresponding structures on the transferred CT or MR images. B-mode and CT or MR images were concurrently displayed on the US monitor for comparison.
B-mode and CEUS images were retrospectively analyzed by a radiologist with 12 years of experience in CEUS, who was blinded to the histopathological results of the target lesions. The radiologist assessed the conspicuity of the entire tumor and the viable tumor on B-mode images for the B-mode group, and on both B-mode and CEUS images for the CEUS group, using a 4-point scale: 1 indicated the tumor was definitely unidentifiable; 2 suggested the tumor was probably identifiable but with low confidence due to poor lesion conspicuity; 3 meant the tumor was identifiable with confidence; and 4 indicated the tumor was definitely identifiable with high confidence [19]. The viable-tumor conspicuity score was determined by examining the presumed solid tumor portion while avoiding areas of hemorrhage, necrosis, or lipiodol uptake. On CEUS, contrast enhancement in a target lesion was considered to indicate a viable tumor.
The pattern and degree of arterial phase enhancement on CEUS images were classified into four categories, relative to the enhancement level of the surrounding liver parenchyma: non-rim hyperenhancement, rim hyperenhancement, isoenhancement, and hypoenhancement. Additionally, the timing and extent of contrast washout were evaluated [20]. Washout occurring within 60 seconds was defined as early washout, while any washout occurring later was termed late washout [20]. Mild washout was described as a scenario in which the lesion exhibited a lower level of enhancement compared to the parenchyma, and marked washout was defined as the lesion appearing almost entirely without contrast within 2 minutes after contrast injection [20].
Additionally, pre-procedural contrast-enhanced CT/MR images were analyzed. Enhancement patterns on arterial phase images, as well as washout patterns, were evaluated for viable HCC portions after treatment in accordance with the Liver Imaging-Reporting and Data System version 2018 [2].
All statistical analyses were performed using IBM SPSS Statistics for Windows version 25.0 (IBM Corp., Armonk, NY, USA) and MedCalc Statistical Software version 18.9.1 (MedCalc Software, Ostend, Belgium). Patient characteristics, whole- and viable-tumor conspicuity scores, and biopsy success rates were compared between the CEUS and B-mode groups. For categorical variables, the chi-square or Fisher exact test were utilized, while for continuous variables, the Mann-Whitney U test was employed. Additionally, CEUS imaging features of HCC and non-HCC lesions were compared using the chi-square test. P-values of less than 0.05 were considered to indicate statistical significance.
The CEUS group comprised 36 participants, with a mean age±standard deviation (SD) of 65.3±9.2 years (male:female ratio, 31:5). The B-mode group included 60 patients, with a mean age±SD of 60.7±12.3 years (male:female ratio, 50:10) (Table 1). No significant difference was found between groups in the mean size of biopsy-targeted lesions (CEUS, 3.8±2.3 cm; B-mode, 3.7±3.3 cm, P=0.332). Lesions were most commonly found in the right anterior segment of the liver in both groups (CEUS, 38.9% [14 of 36]; B-mode, 53.3% [32 of 60]; P=0.396). The majority of patients in both groups received an initial diagnosis of HCC based on noninvasive criteria (CEUS, 69.4% [25 of 36]; B-mode, 83.3% [50 of 60]; P=0.131) [1]. No significant difference was observed in the proportion of patients for whom fusion techniques were used (CEUS, 11.1% [4 of 36]; B-mode, 20.0% [12 of 60]; P=0.397) or in the number of biopsy cores obtained per patient (CEUS, 2.8±0.6; B-mode, 2.7±0.7; P=0.318). No technical failures or severe misregistrations occurred during the application of fusion techniques that would have interrupted the biopsy procedures.
Regarding arterial enhancement, washout, and diffusion restriction, no significant differences were observed between the CEUS and B-mode US groups (all P-values >0.05) (Table 2).
The conspicuity scores for both the whole tumor and the viable tumor, as assessed by B-mode US, did not differ significantly between the CEUS and B-mode groups (whole tumor: 1.8±0.9 vs. 1.9±0.7, respectively [P=0.458]; viable tumor: 1.4±0.8 vs. 1.5±0.7, respectively [P=0.201]). However, both whole- and viable-tumor conspicuity scores were significantly higher on CEUS than on grayscale US within the CEUS group (whole tumor: 2.7±0.5 vs. 1.8±0.9, P<0.001; viable tumor: 2.6±0.7 vs. 1.4±0.8, P<0.001).
In the CEUS group (n=36), histopathologic analysis of 32 participants revealed the following diagnoses: HCC in 27 cases (84.4%), neuroendocrine tumor grade 3 in one case (3.1%), neuroendocrine tumor grade 2 in one case (3.1%), high-grade dysplastic nodule in one case (3.1%), combined HCC-cholangiocarcinoma (CCA) in one case (3.1%), and undifferentiated carcinoma in one case (3.1%). The biopsy specimens from the remaining four participants yielded non-diagnostic results. Therefore, the biopsy success rate in the CEUS group was 88.9% (32 of 36 cases). In the B-mode group (n=60), confirmative histopathologic analysis of 42 patients identified HCC in 37 cases (88.1%), combined HCC-CCA in three cases (7.1%), poorly differentiated carcinoma in one case (2.4%), and diffuse large B-cell lymphoma in one case (2.4%). The remaining 18 patients had non-diagnostic liver biopsy results. The biopsy success rate in the CEUS group (88.9%; 32 of 36 cases) was significantly higher than the rate observed in the B-mode group (70.0% [42 of 60 cases], P=0.044). No biopsy-related complications arose in either group. A representative case is illustrated in Fig. 2. The characteristics of participants with non-HCC lesions are provided in the supplementary data.
Regarding the biopsy success rate and the pattern of arterial phase enhancement of the target lesion, no significant differences were noted in the biopsy success rate between patients with non-rim arterial phase hyperenhancement and those with rim hyperenhancement or hypo/isoenhancement in the CEUS group (92.9% [26 of 28 cases] vs. 75.0% [6 of 8 cases], P=0.162).
Regarding the pattern and degree of arterial enhancement, both HCC and non-HCC lesions most frequently exhibited non-rim hyperenhancement, with no significant difference observed between them (HCC, 85.2% [23 of 27 lesions]; non-HCC, 60.0% [3 of 5 lesions]; P=0.388) (Table 3). In terms of washout characteristics, HCC lesions showed a significantly higher incidence of late mild washout compared to non-HCC lesions (92.6% [25 of 27 lesions] vs. 60.0% [3 of 5 lesions], respectively; P=0.036). None of the HCC lesions displayed a marked washout pattern, while one combined HCC-CCA lesion among the five lesions in the non-HCC group exhibited such a pattern (20.0%) (Fig. 3). As for early washout, it was observed in only two of the 27 HCC lesions (7.4%) and in one neuroendocrine tumor grade 2 among the five non-HCC lesions (20.0%).
The diagnostic results exhibited a late mild washout pattern more frequently than the non-diagnostic results (87.5% [28 of 32] vs. 25.0% [1 of 4], P=0.004) (Table 4). Regarding the arterial enhancement pattern, no significant difference was observed between these two subgroups (P=0.415).
Patients with biopsy-confirmed HCC from the CEUS group (27 of 36, 75.0%) and the B-mode US group (37 of 60, 61.7%) received systemic therapy, were enrolled in clinical trials, or underwent TACE, radiotherapy, or RFA (Table 5). For those with biopsy-confirmed non-HCC lesions in the CEUS group (5 of 36, 13.9%), treatments included chemotherapy for neuroendocrine tumor grade 3 and combined HCC-CCA, clinical trial enrollment for neuroendocrine tumor grade 2, and regular follow-up for high-grade dysplastic nodule. One patient with undifferentiated carcinoma received conservative treatment due to a deteriorating systemic condition. In the B-mode group, patients with biopsy-confirmed non-HCC lesions (5 of 60, 8.3%) were treated with chemotherapy for combined HCC-CCA and diffuse large B-cell lymphoma, or with RFA for poorly differentiated carcinoma. To inform treatment planning, re-biopsy was conducted for patients with non-diagnostic initial results from both the CEUS (2 of 36, 5.6%) and B-mode (9 of 60, 15.0%) groups. One patient of the 36 in the CEUS group (2.8%) and nine patients of the 60 in the B-mode group (15.0%) received further treatment for HCC based on a presumed diagnosis, as they received non-diagnostic results.
In this study, the success rate of CEUS-guided liver biopsy for treated HCC was 88.9%, with 15.6% (5 of 32) of the patients being diagnosed with non-HCC lesions. These results suggest that CEUS-guided biopsy could be beneficial for obtaining tissue specimens for targeted therapy in patients with treated HCC.
The findings demonstrate that the diagnostic yield of B-mode image-guided liver biopsy was 70.0%, with a mean tumor size of 3.7 cm. This yield is considerably lower than the 85.1%-94.5% reported in previous studies, which focused on treatment-naïve lesions with mean tumor sizes ranging from 4.2 to 4.5 cm [11,12]. The discrepancy in diagnostic yields between the present study and previous research may be attributed to the challenging task of differentiating viable tissue from necrosis or treatment-related changes in B-mode images following prior treatment for HCC [21]. Indeed, in the present study, both whole- and viable-tumor conspicuity scores on B-mode US were as low as 1.4-1.8 for both CEUS and B-mode groups. These scores indicate that the tumors were either "definitely unidentifiable" or "probably identifiable, but with low confidence due to poor lesion conspicuity." The suboptimal conspicuity of viable HCC, coupled with the fact that most participants in both the CEUS and B-mode groups had undergone TACE, likely contributed to the lower diagnostic yields in the present study compared to those found in previous research. In this context, CEUS offers advantages over B-mode imaging in detecting viable tumors and assessing tumor vascularity, thereby facilitating targeted biopsy [16,22,23]. In this study, the conspicuity scores for both whole and viable tumors on CEUS were significantly higher than those on grayscale US within the CEUS group, which may have resulted in an increased biopsy yield. These findings are consistent with previous research demonstrating the high accuracy of CEUS in identifying residual tumors following various locoregional treatments [16,22].
Patients with a confirmed diagnosis through liver biopsy received various treatments corresponding to the histopathologic findings. In contrast, 5.6% of patients in the CEUS group (2 of 36) and 15.0% of patients in the B-mode group (9 of 60) required re-biopsy due to non-diagnostic initial liver biopsy results. These findings suggest a cautious conclusion that CEUS-guided biopsy may reduce the need for re-biopsy in treated HCC cases. Repeat biopsy is associated with an increased risk of complications, such as bleeding [24], that can impact the therapeutic pathway; therefore, efforts to increase the success rate of biopsy, including the adoption of CEUS guidance, are crucial. Additionally, CEUS-guided biopsy for treated HCC may result in a more definitive treatment plan compared to B-mode-guided biopsy, due to its higher success rate. In the present study, only one patient in the CEUS group continued HCC treatment based on a presumptive diagnosis following a non-diagnostic biopsy result. In contrast, over 10% of patients in the B-mode group continued HCC management based on a presumptive diagnosis.
The present study had several limitations. First, the CEUS group included a relatively small number of patients. Second, selection bias may have been unavoidable in the B-mode group, which served as a historical control, due to its retrospective enrollment. Nevertheless, the two groups demonstrated no significant differences in baseline characteristics, such as mean tumor size or location.
In conclusion, the diagnostic yield of CEUS-guided liver biopsy for treated HCC was 88.9%, which was significantly higher than that of B-mode image-guided biopsy. Additionally, CEUS-guided liver biopsy led to the diagnosis of non-HCC lesions in 15.6% of patients.
NotesAuthor Contributions Conceptualization: Lee DH. Data acquisition: Yoo J, Lee DH. Data analysis or interpretation: Yoo J. Drafting of the manuscript: Yoo J. Critical revision of the manuscript: Lee DH. Approval of the final version of the manuscript: all authors. Conflict of InterestThis work was supported by a research grant from Canon Medical Systems Korea (No. 2019-01), awarded to D. H. Lee. D. H. Lee serves as Editor for the Ultrasonography, but has no role in the decision to publish this article. J. Yoo has no conflicts of interest. AcknowledgementsThis work was supported by a research grant from Canon Medical Systems Korea (No. 2019-01), awarded to D. H. Lee.
References1. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182–236.
2. Chernyak V, Fowler KJ, Kamaya A, Kielar AZ, Elsayes KM, Bashir MR, et al. Liver Imaging Reporting and Data System (LI-RADS) version 2018: umaging of hepatocellular carcinoma in at-risk patients. Radiology 2018;289:816–830.
3. Korean Liver Cancer Association (KLCA); National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Clin Mol Hepatol 2022;28:583–705.
4. Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 2017;11:317–370.
5. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358–380.
6. Di Tommaso L, Spadaccini M, Donadon M, Personeni N, Elamin A, Aghemo A, et al. Role of liver biopsy in hepatocellular carcinoma. World J Gastroenterol 2019;25:6041–6052.
7. Childs A, Zakeri N, Ma YT, O'Rourke J, Ross P, Hashem E, et al. Biopsy for advanced hepatocellular carcinoma: results of a multicentre UK audit. Br J Cancer 2021;125:1350–1355.
8. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 2020;382:1894–1905.
9. Sangro B, Sarobe P, Hervas-Stubbs S, Melero I. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2021;18:525–543.
10. Elsayes KM, Ellis JH, Elkhouly T, Ream JM, Bowerson M, Khan A, et al. Diagnostic yield of percutaneous image-guided tissue biopsy of focal hepatic lesions in cancer patients: ten percent are not metastases from the primary malignancy. Cancer 2011;117:4041–4048.
11. Kang JH, Choi SH, Kim SY, Lee SJ, Shin YM, Won HJ, et al. US LI-RADS visualization score: diagnostic outcome of ultrasound-guided focal hepatic lesion biopsy in patients at risk for hepatocellular carcinoma. Ultrasonography 2021;40:167–175.
12. Kim DW, Kim SY, Kang HJ, Kang JH, Lee SS, Shim JH, et al. Diagnostic performance of ultrasonography-guided core-needle biopsy according to MRI LI-RADS diagnostic categories. Ultrasonography 2021;40:387–397.
13. Kim JH, Eun HW, Kang HJ, Lee SM, Han JK. Clinical usefulness of contrast-enhanced ultrasound for percutaneous biopsies of focal liver lesions. Ultrasound Med Biol 2019;45:S99.
14. Partovi S, Lu Z, Kessner R, Yu A, Ahmed Y, Patel IJ, et al. Contrast enhanced ultrasound guided biopsies of liver lesions not visualized on standard B-mode ultrasound-preliminary experience. J Gastrointest Oncol 2017;8:1056–1064.
15. Cao X, Liu Z, Zhou X, Geng C, Chang Q, Zhu L, et al. Usefulness of real-time contrast-enhanced ultrasound guided coaxial needle biopsy for focal liver lesions. Abdom Radiol (NY) 2019;44:310–317.
16. Hai Y, Savsani E, Chong W, Eisenbrey J, Lyshchik A. Meta-analysis and systematic review of contrast-enhanced ultrasound in evaluating the treatment response after locoregional therapy of hepatocellular carcinoma. Abdom Radiol (NY) 2021;46:5162–5179.
17. Vernuccio F, Rosenberg MD, Meyer M, Choudhury KR, Nelson RC, Marin D. Negative biopsy of focal hepatic lesions: decision tree model for patient management. AJR Am J Roentgenol 2019;212:677–685.
18. Ewertsen C, Saftoiu A, Gruionu LG, Karstrup S, Nielsen MB. Real-time image fusion involving diagnostic ultrasound. AJR Am J Roentgenol 2013;200:W249–W255.
19. Rhim H, Choi D, Kim YS, Lim HK, Choe BK. Ultrasonography-guided percutaneous radiofrequency ablation of hepatocellular carcinomas: a feasibility scoring system for planning sonography. Eur J Radiol 2010;75:253–258.
20. Kim TK, Noh SY, Wilson SR, Kono Y, Piscaglia F, Jang HJ, et al. Contrast-enhanced ultrasound (CEUS) liver imaging reporting and data system (LI-RADS) 2017: a review of important differences compared to the CT/MRI system. Clin Mol Hepatol 2017;23:280–289.
21. Kang HJ, Lee JM, Jeon SK, Ryu H, Yoo J, Lee JK, et al. Microvascular flow imaging of residual or recurrent hepatocellular carcinoma after transarterial chemoembolization: comparison with color/power Doppler imaging. Korean J Radiol 2019;20:1114–1123.
22. Watanabe Y, Ogawa M, Kumagawa M, Hirayama M, Miura T, Matsumoto N, et al. Utility of contrast-enhanced ultrasound for early therapeutic evaluation of hepatocellular carcinoma after transcatheter arterial chemoembolization. J Ultrasound Med 2020;39:431–440.
Flowchart depicting the process of patient enrollment.CEUS, contrast-enhanced ultrasound; HCC, hepatocellular carcinoma; US, ultrasound.
 Fig. 1.CEUS-guided liver biopsy in a 67-year-old man who underwent TACE and radiation therapy for HCC.A. An axial arterial phase image from a contrast-enhanced CT scan reveals a 2-cm arterial enhancing region within the mass, which also exhibits partial lipiodol uptake from previous TACE (arrows); this finding suggests the presence of viable HCC. B. An arterial phase CEUS image (left), acquired 22 seconds after the intravenous administration of contrast medium, clearly visualizes the viable tumor, which exhibits arterial phase hyperenhancement (arrows) distinct from the non-enhancing, treated area (arrowhead); a B-mode image (right) shows both the viable tumor and the treated area as a heterogeneously hypoechoic lesion. C. The viable tumor (arrows) exhibits late mild washout at 3 minutes and 10 seconds following the administration of contrast medium. D. A biopsy was performed on the viable portion of the tumor (arrows), and the histopathological findings reveal HCC. CEUS, contrast-enhanced ultrasound; TACE, transarterial chemoembolization; HCC, hepatocellular carcinoma; CT, computed tomography.
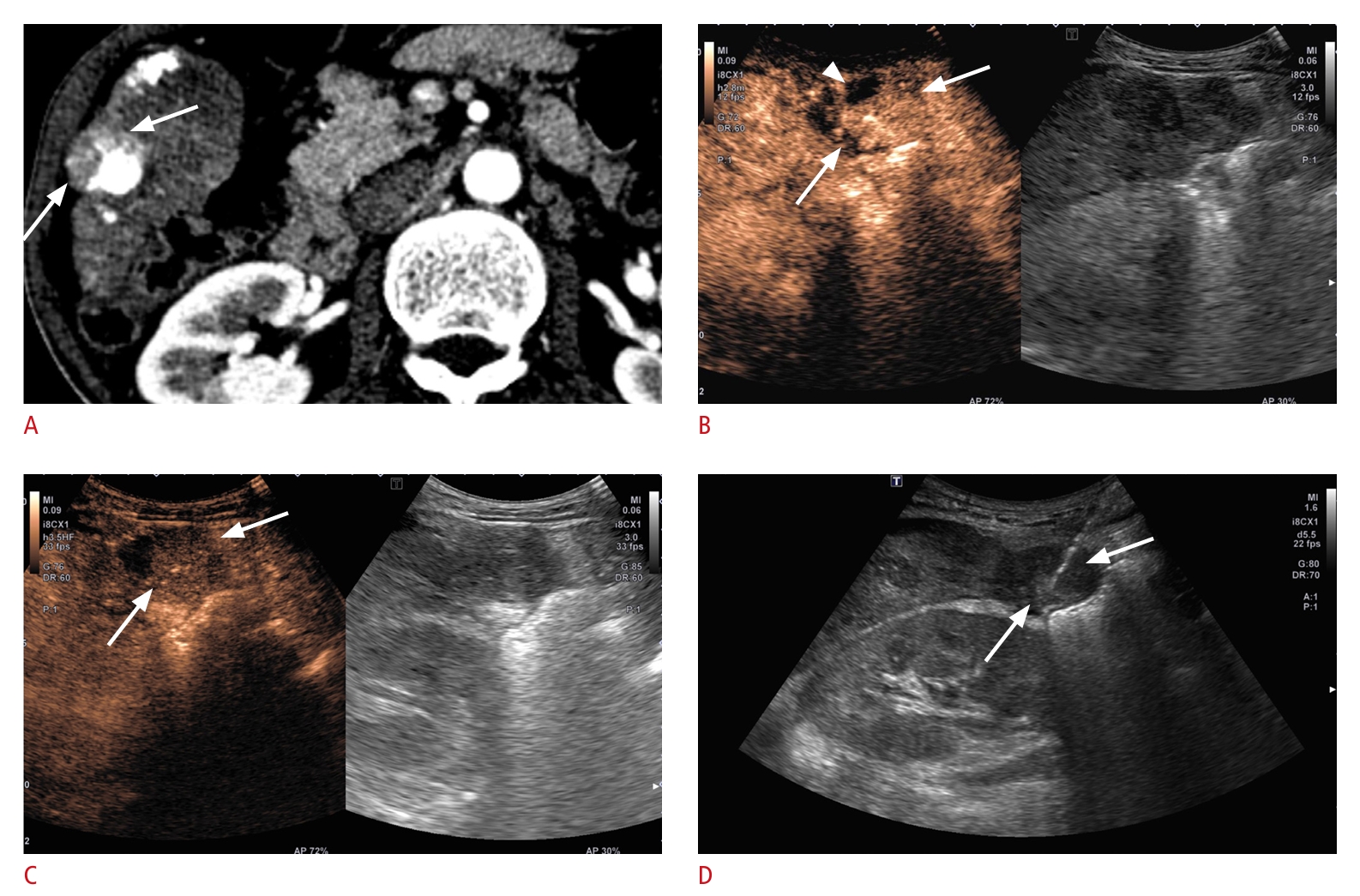 Fig. 2.CEUS-guided liver biopsy in a 52-year-old man who underwent TACE for HCC.A. An axial arterial phase image from a contrast-enhanced CT scan reveals an area of arterial enhancement within the necrotic region resulting from prior treatment (arrows), suggesting the presence of viable HCC. B. An arterial phase CEUS image (left), acquired 30 seconds after the intravenous administration of contrast medium, reveals a viable tumor exhibiting arterial phase hyperenhancement (arrows). C. The viable tumor (arrows) displays marked washout within 2 minutes following the administration of contrast medium. D. Biopsy was performed on the viable portion of the tumor (arrows), and the histopathological findings reveal a combined HCC-cholangiocarcinoma. CEUS, contrast-enhanced ultrasound; TACE, transarterial chemoembolization; HCC, hepatocellular carcinoma; CT, computed tomography.
 Fig. 3.Table 1.Patient characteristics
Table 2.Comparison of imaging features of viable tumors on pre-procedural CT/MRI in CEUS and B-mode groups
Table 3.Comparison of imaging features between biopsytargeted HCC lesions and non-HCC lesions in the CEUS group Table 4.Comparison of imaging features between diagnostic and non-diagnostic biopsy results in the CEUS group Table 5.Management after biopsy in CEUS and B-mode groups |



 Print
Print facebook
facebook twitter
twitter Linkedin
Linkedin google+
google+

 Download Citation
Download Citation PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI




