Introduction
Several key concepts encapsulate the future of medicine, including early diagnosis using molecular imaging, personalized medicine using genetic data, minimally invasive surgery with advanced surgical techniques and medical devices, and image-guided therapy using imaging modalities and newly developed therapeutic materials.
The most common imaging technique is X-rays. Ultrasonography is the second most commonly used imaging technique in clinical contexts. It is non-invasive, widely available, portable, relatively inexpensive, and allows real-time imaging without the use of ionizing radiation [1-4].
Ultrasonography has become a cornerstone for diagnosis and patient management. A new field, known as image-guided therapeutics delivery, harnesses the vast potential of ultrasound technologies for treatment delivery, and promises to fulfill the vision of personalized medicine [2]. Many researchers are trying to create complexes that target specific cells and deliver therapeutic materials into those cells. The prostate is a very good candidate for exploring these possibilities, especially using ultrasonography.
Prostate cancer is one of the important diseases in the world. It is the most common neoplasm in Europe and America, with an incidence two to three times that of lung and colorectal cancer [5]. Its incidence is still rising in Asian countries, including Japan and Korea. The screening, detection, and diagnosis of prostate cancer are currently based on serum prostate-specific antigen levels, digital rectal examinations, and transrectal ultrasound-guided systematic biopsies [5].
Ultrasound plays a major role in the diagnosis of prostate cancer. With advances in research into targeted imaging, many studies are being performed to detect early prostate cancer through targeted imaging. This review describes the usage and design of ultrasound-contrast complexes for visualizing prostate cancer and for delivering therapeutic materials in the context of prostate cancer management.
Microbubbles
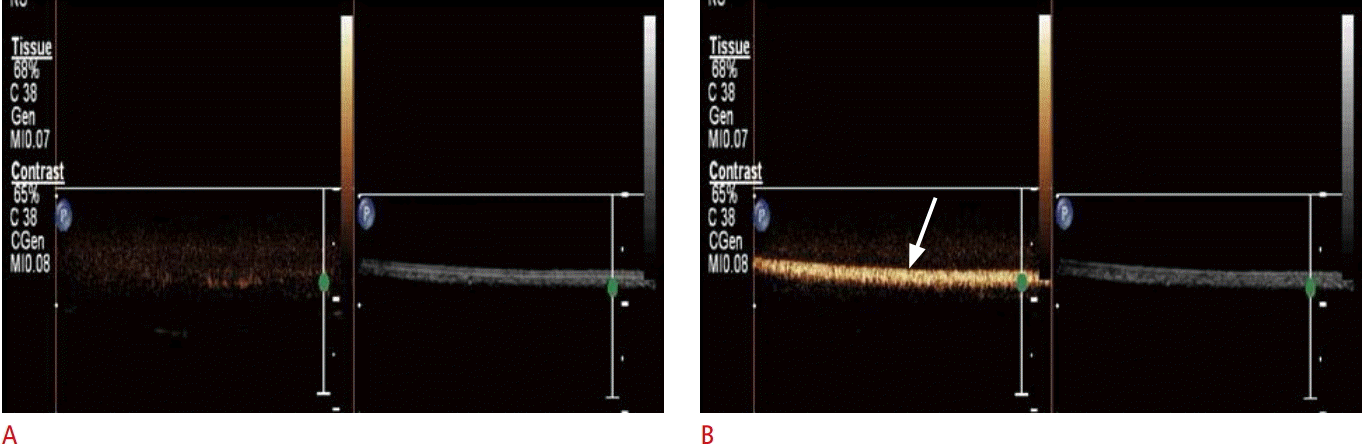
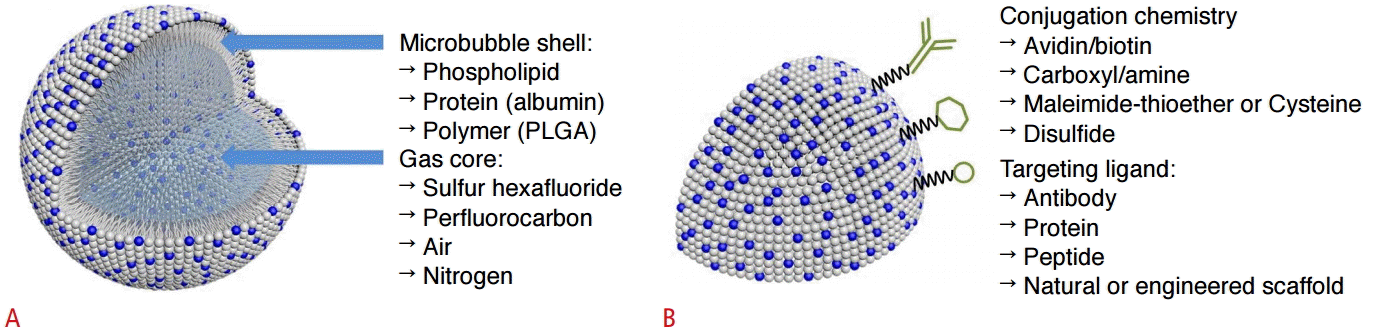
The most commonly used ultrasound molecular-imaging contrast agents are microbubbles, which range in size from 1 to 8 μm. They consist of a gas core stabilized by a surrounding shell made from materials such as phospholipids, biocompatible polymers, or proteins [1-4]. In a previous study, we prepared microbubbles with a spherical shape and a mean diameter of about 2 μm, and a narrow size distribution (Fig. 1). The inner gases used in microbubbles are ordinary air, nitrogen, or biologically inert heavy gases, including perfluoropropane, perfluorobutane, perfluorohexane, or sulfur hexafluoride [1-5]. Heavy gases usually show better stability and lead to a longer blood half-life of the microbubbles. Yoon et al. [6] and Moon et al. [7] described the preparation and function of microbubbles in detail. We experimentally confirmed the echogenicity of the microbubbles. Compared with phosphate-buffered saline (Fig. 2A), microbubbles show high echogenicity, which makes them useful as an ultrasound contrast agent (arrow in Fig. 2B). The synthesis of targeted microbubbles is not significantly different from the synthesis of non-targeted microbubbles, except for an additional process that adds binding ligands to the shell of the microbubbles.
The microbubbles can act as cavitation nuclei that carry drugs or genes to target cells, enabling site-targeted treatment [8-10]. Ultrasonography can facilitate microbubble-mediated delivery by inducing the collapse of the microbubbles, perforations in the cell membranes, and increasing the permeability of regional capillaries, thereby allowing large molecules to stream into the cells. This phenomenon, known as sonoporation, can enhance the intracellular delivery of drugs or genes in ultrasound microbubble-mediated delivery [9,11]. When the microbubbles are injected intravenously, the majority of microbubbles are cleared by the hepatic and splenic reticuloendothelial system (RES), with 95% of the microbubbles cleared from the blood pool after 30 minutes [12]. The gas contained within the microbubbles is removed from the body by exhalation [4].
Low mechanical index (MI) imaging (MI of 0.1 or less) allows the visualization of microbubbles as a contrast agent without destroying them due to nonlinear oscillations [13]. These asymmetric nonlinear oscillations result in the generation of harmonic (second harmonic and above) or subharmonic (half of the center frequency) echoes that can be leveraged to enhance the signal-to-noise ratio from the microbubbles in comparison to the surrounding tissue. These echoes can be visualized using a range of contrast imaging technologies, such as pulse inversion or amplitude modulation [14].
Targeting Strategy
Active and passive targeting strategies have been developed. Passive targeting takes advantage of enhanced permeability and retention [15-17]. However, this phenomenon, which has been primarily observed in subcutaneous xenograft mouse tumors, is often muted in other models, including orthotopic animal models in mice or larger species [17].
The other targeting strategy is active targeting. Several conjugation techniques exist to couple ligands to the bubbles. The targeting ligands include antibodies, peptides, and natural or engineered scaffolds, and they can be directly incorporated into the shell during or after the microbubble synthesis process [4]. In order to attach the ligands to the microbubble shell, a suitable reactive moiety, such as biotin, a carboxylate group, thiol, or maleimide, is attached to a shell-forming material component. The avidin-biotin interaction is among the strongest noncovalent bonds and is widely used in biomedical research and analytic techniques such as immunoassays (Fig. 3). However, foreign proteins such as avidins can cause an immune response, and this coupling scheme is therefore useful only in preclinical research. Antibodies have clinical limitations due to the immune response. In order to avoid this issue, antibodies must be adapted for use in humans.
PEGylation, using polyethylene glycol (PEG), has been used to create so-called stealthy agents and reduce RES clearance rates, but it can impair ligand-directed targeting due to steric interference. Moreover, PEG, once thought to be a benign surface modifier because it reduces complement activation, has been found to induce adaptive immune responses with repeated usage [18]. In active targeting, the homogeneous and highly restricted expression of tumor-associated antigens is critical to ensure the success of targeted immunotherapies. Much research is currently focused on the recognization of materials related to angiogenesis due to tumors and biomarkers specific to tumors.
Vascular Endothelial Growth Factor Receptor Type 2
Recently, the first targeted molecular ultrasound contrast agent has been designed and entered clinical trials in humans [19]. This contrast agent, known as BR55 (Bracco, Milano, Italy), consists of a gas core composed of a mixture of perfluorobutane and nitrogen, and is surrounded by a phospholipid shell with a mean diameter of 1.5 μm [4,20,21]. The ligand is attached at the kinase insert domain receptor (KDR; the human analog of vascular endothelial growth factor receptor type 2 [VEGFR2]). VEGFR2 is overexpressed in the neovasculature of many human cancer types, including prostate cancer, breast cancer, ovarian cancer, pancreatic cancer, and colorectal cancer.
Prostate-Specific Membrane Antigen
Prostate-specific membrane antigen (PSMA) is an attractive target for detecting primary and metastatic prostate cancer because its expression is elevated throughout all stages of the progression of prostate cancer, and it is highly restricted to prostate cells, with very low levels of expression detectable in other tissues [22]. PSMA, also known as folate hydrolase I, glutamate carboxypeptidase II, and N-acetyl-L-aspartyl-L-glutamate peptidase I, is a 750-amino acid type II transmembrane glycoprotein that is primarily expressed in normal human prostate epithelium and is elevated in prostate cancer, including metastatic disease [23].
It has been reported that elevated levels of PSMA in patients with primary prostate cancer correlate with other traditional adverse prognostic factors and independently predict disease outcomes [24].
Since PSMA is expressed in all prostate cancers and its expression is further increased in poorly differentiated, metastatic, and hormone-refractory carcinomas, it is a very attractive target for the diagnosis, staging, and treatment of the disease [25-27].
Recently, an indium-111 radiolabeled anti-PSMA monoclonal antibody (Capromab Pendetide, Cytogen Corporation, Princeton, NJ, USA) has been used to detect soft-tissue metastasis and recurrence of prostate cancer. This antibody targets the intracellular domain of PSMA and is thought to bind mostly to the necrotic cells of prostate tumors [28]. More recently, Nawaz [29]. have developed and radiolabeled monoclonal antibodies that bind to the extracellular domain of PSMA, and these antibodies have entered into multiple clinical trials, including one exploring targeted radiotherapy for metastatic prostate cancer. Several PSMA monoclonal antibodies have been used as vehicles for the targeted delivery of cytotoxic agents, including radioactive isotopes, small molecules, and protein toxins [30-33].
Although monoclonal antibodies have potential as tumor-targeting agents, their long circulating half-life and poor ability to penetrate tumors limit their effectiveness as diagnostic and therapeutic agents [34]. Due to these limitations, monoclonal antibodies have shown only limited clinical success to date, mostly in the treatment of blood-borne cancers, such as non-Hodgkin lymphoma [35].
Small molecules such as peptides, vitamins, and substrate analogs offer significant advantages over antibodies for targeting solid tumors [36]. They exhibit better tumor penetration than antibodies [37]. Since small molecules exhibit enhanced diffusibility into the extravascular space and faster blood clearance than antibodies, they show a lower background signal. Moreover, synthesizing analogs that exhibit diverse chemical properties may allow the binding affinity and pharmacokinetics to be altered. A series of small-molecule PSMA inhibitors with high affinity (in the low nanomolar range) and selectivity also have been explored for the imaging of PSMA-positive prostate cancer cells [33,38-40].
Human Epidermal Growth Factor Receptor 2
Human epidermal growth factor receptor 2 (HER2) is associated with the expression of the ErbB-2, CD340, and p185 genes. The amplification or overexpression of ErbB-2 occurs in approximately 30% of breast cancers, as well as in other tumors. Since it is well known that some kinds of prostate cancer cells express HER2 [41], researchers are trying to target prostate cancer cells by conjugating anti-HER2 antibodies to microbubbles. In one such study, the intracellular uptake of the microbubble-anti-HER2 antibody complex was significantly higher in LNCaP cells, which express higher levels of HER2, than in PC-3 cells. This proved that microbubble complexes conjugated with anti-HER2 antibodies were capable of effectively targeting prostate cancer cells [41].
Integrin
Integrins consist of noncovalently associated α and β subunits, and are cell surface receptors that primarily mediate the interactions of cells with components of the extracellular matrix. It has been well established that αvβ3 integrin is usually expressed at low or undetectable levels in normal cells but can be highly elevated in most cancer cells and especially in prostate cancer cells [42-44]. This protein is known to contribute to the migration, proliferation, and survival of cancer cells. Moreover, αvβ3 integrin is also involved in the pathogenesis of bone metastases [45] and has been found to play a crucial role in bone metastasis [46]. A drug delivery system conjugating Arg-Gly-Asp (RGD) peptides for targeting αvβ3 integrin has been also documented in animal models of primary tumors [44,47,48].
Several preclinical and clinical studies have been performed to image primary prostate cancer and/or metastasis using radiolabeled RGD peptides and analogs targeting integrin αvβ3 for tumor angiogenesis imaging and tumor therapy [44,49-53]. αvβ3 and GRPr are validated biomarkers present on the surfaces of most prostate cancer cells. Micro-positron emission tomography imaging investigations at 4 hours after injection produced high-quality and high-contrast whole-body images with minimal tracer present in surrounding collateral abdominal tissues. The high selectivity and retention of this tracer in tumor tissue suggest that a 67-Cu radiolabeled agent of this type may also be useful in targeted radiotherapy for primary prostate cancer tumors and metastatic disease.
Furthermore, the results of the above study support those of previous studies published by other research groups, which have suggested radiolabeled antagonists to be preferable over agonists for the molecular imaging of human cancers.
Delivery of Therapeutics
While ultrasound is a well-established diagnostic imaging technique, its great potential to enhance drug delivery was only recognized in the late 1990s [52]. Since then, the ultrasound-mediated uptake of small drugs, proteins, and larger nanoparticles such as gene complexes or drug-loaded liposomes has been reported [54-59]. Due to the compressibility of microbubbles, microbubbles can cavitate in an ultrasonic field. Cavitation is the alternating shrinking and expanding of the microbubbles according to the pressure phases of the ultrasound wave [60]. Cavitation produces backscatter of the ultrasound waves, thereby intensifying the reflected signal [61]. Since microbubbles amplify the biophysical effects of ultrasound waves, they are also essential for drug delivery using ultrasound. According to the ultrasound intensity, the cavitation of microbubbles may be classified as stable and inertial.
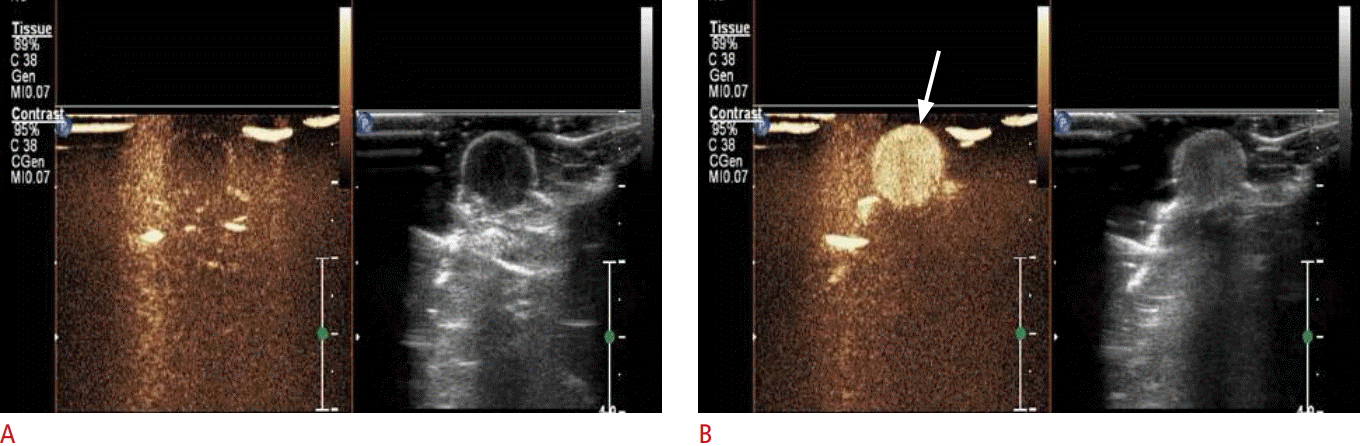
In stable cavitation, microbubbles show continuous low-amplitude oscillations, generating microstreams in the surrounding fluid. Our group has researched the in vivo echogenicity of microbubbles. A xenograft PC-3 tumor model was observed using a clinical ultrasound device. A post-contrast ultrasound image (arrows in Fig. 4B) shows definitively enhanced vascularity compared to before the contrast injection (Fig. 4A). Under higher ultrasound intensities, inertial cavitation occurs, with larger oscillations and eventually bursting of the microbubble. Inertial cavitation is accompanied by more violent phenomena, such as microjets and shock waves resulting in pore formation in the cell membrane, which is known as sonoporation [62]. In addition to sonoporation, enhanced endocytosis also contributes to ultrasound-mediated delivery. The two mechanisms in the ultrasound environment seem to depend on molecule size and the acoustic pressure used.
Although microbubble cavitation in ultrasonic fields has been studied extensively, the biophysical mechanisms leading to enhanced drug delivery are still a matter of debate. Sonoporation is generally accepted as the main mechanism through which drugs enter cells during ultrasound application. Scanning electron microscopy images have shown clear membrane disruptions after ultrasound exposure [63]. Moreover, several studies have demonstrated the uptake of cell-impermeable molecules when cells are exposed to ultrasound [59,64-66].
However, Meijering et al. [67] argued that in addition to pore formation, enhanced endocytosis also contributes to ultrasound-mediated delivery. According to their findings, molecule size determined the route of uptake, with endocytosis playing a greater role for larger molecules. Some other studies have confirmed that endocytosis is indeed involved in ultrasound-mediated uptake [59,68-71]. De Cock et al. [59] hypothesized that this discrepancy in the literature may be due to variations in the intensity levels that have been used in previous studies. In the first part of their study, they investigated whether the route of uptake (via pores or via endocytosis) was dependent on the acoustic pressure used. Moreover, the uptake of low- and high-molecular-weight dextrans was compared to evaluate the role of molecule size. In the second part of their study, they performed real-time confocal microscopy during ultrasound radiation to reveal microbubble-cell interactions. They concluded that low acoustic pressure enhanced uptake by predominantly stimulating endocytosis, whereas high acoustic pressures led to uptake via membrane pores. The primary radiation force propelled microbubbles towards cells at a high velocity. Upon collision, pores were created in the cell membrane. When designing drug delivery experiments, these findings should be considered in order to select the optimal ultrasound settings [59].
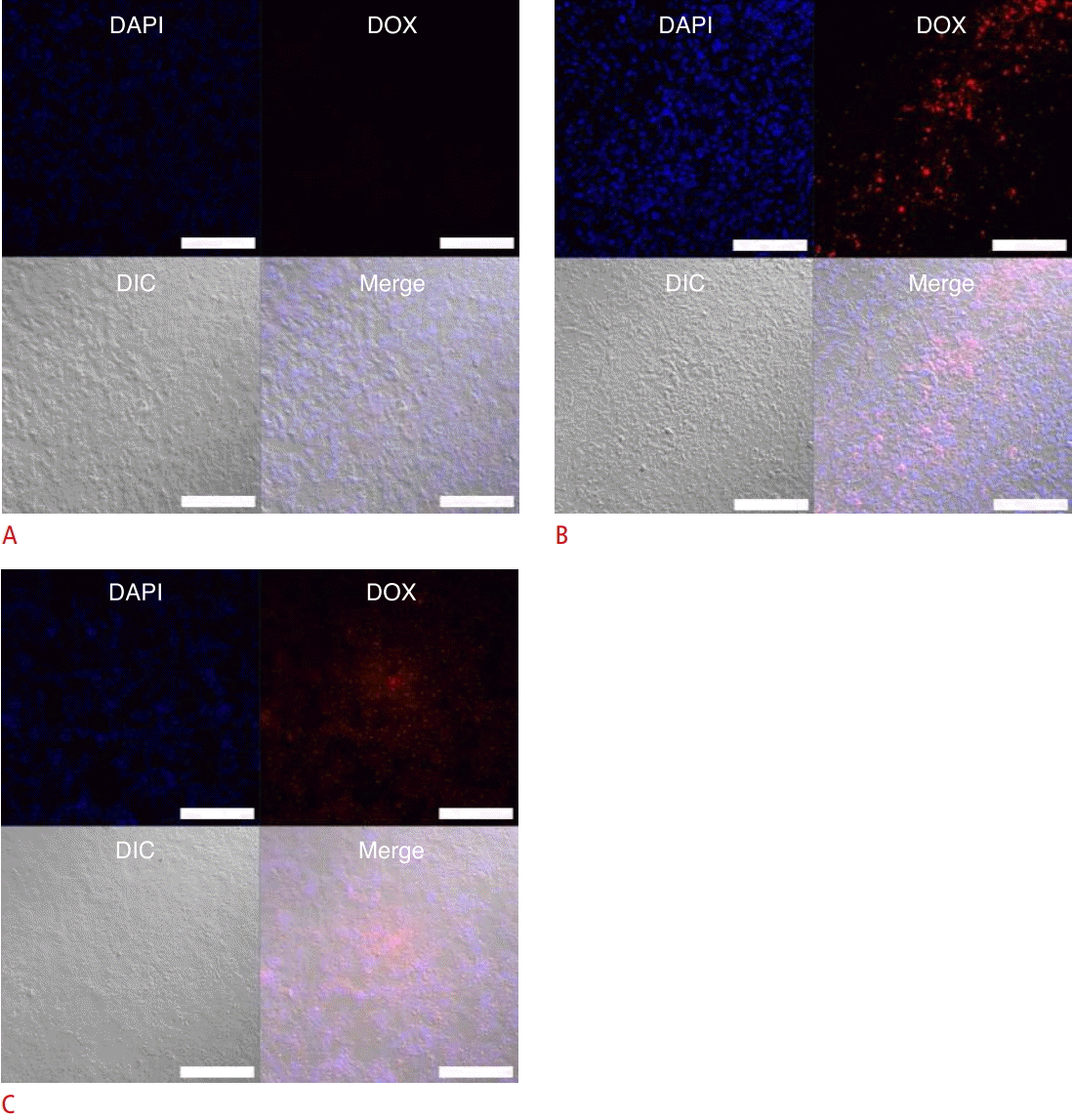
Our group has researched the delivery effect of sonoporation in an in vivo model (Fig. 5). In order to confirm the delivery of anticancer drugs into tumor tissues within a xenograft PC-3 tumor model, a confocal laser scanning microscope was used for observation. Microbubbles with doxorubicin were injected via the tail vein, and then ultrasound treatment for delivery was performed using a sonoporator (Sonidel, Dublin, Ireland). Changing the treatment time from 5 minutes to 15 minutes increased the fluorescence intensity of doxorubicin in tumor tissues.
Recently, in addition to drug delivery, new therapeutic methods have been introduced for treating prostate cancer, including RNA interference (RNAi). RNAi relies on post-transcriptional gene silencing using double-strand RNA processed into strands of 21-25 nucleotides, known as small interfering RNA (siRNA) [72]. New targeted gene therapy using RNAi is being investigated for the treatment of prostate cancer. siRNA promotes targeted gene silencing by the sequence-specific degradation of messenger RNA when it is incorporated into RNA-induced silencing complexes in the cytoplasm [72,73]. Since siRNA can prevent the production of specific proteins essential for the proliferation of tumor cells, it is a promising therapeutic approach for cancer treatment.
Conclusion
One of the most common modalities used in prostate imaging is transrectal ultrasonography. The role of ultrasonography in evaluating the prostate may no longer be limited to imaging, but may expand to incorporate image-guided therapy using microbubbles. Several targeting strategies are being researched, with a focus on many specific ligands, including PSMA, integrin, VEGFR2, and HER2, which may be promising examples of targeting. Ultrasound molecular imaging has just started its long journey from in vitro and in vivo preclinical studies to the first human clinical trials. Of particular note, the safety and convenience of ultrasound make it an attractive imaging modality for clinical applications. Ultrasound-mediated delivery has other advantages, such as the simultaneous delivery of anti-cancer drugs and genetic therapeutics. The use of ultrasound and microbubble complexes containing genetic therapeutics and chemotherapeutic agents creates the possibility of clinically applicable image-guided therapy and provides novel prospects for therapeutic applications in the near future.



 Print
Print facebook
facebook twitter
twitter Linkedin
Linkedin google+
google+

 Download Citation
Download Citation PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC




